Materials and MFG Process Exam 1 Notes
1/134
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
135 Terms
metals are?
high stiffness, low elasticity, high strength, ductility, good conductive of heat and electricity.
example of metal?
Steel, Casting irons, alloys
Polymers are?
low strength, low density, low melting point, low conductive of heat and electricity.
example of polymers?
PE,PP,PET, nylons, polyesters, expoxies
Ceramics are?
Low elasticity, high strength, high hardness, brittle, high melting point, low conductive of heat and electricity.
example of ceramics?
Aluminas, silicon carbides, silicon nitrides, zirconias
Glasses are?
Low tensile strength, brittle, transparent and corrosion resistant.
example of glasses?
soda glass, silica glass, glass ceramics
Elastomers are?
highly amorphous materials, high randomly orientated structure.
example of elasomers?
Isoprene, butyl rubber, natural rubber, silicones, EVA
Hybrids?
Composites sandwiches, segmented structures lattices and forams.
Density
mass divided volume
Ductility
the amount a material can change length/shape before fracture (how "stretchy" it is)
Strength
the ability to withstand loads without fracture (how high of stress it can withstand)• A material property (e.g., yield stress, ultimate tensile stress)
Stiffness
how much a material/structure deflects under a given load•
Based on both the intrinsic material properties (stress vs. strain behavior) and the geometric features
Example of Stiffness
an I-beam is several times stiffer than a square beam made of the same type/amount of material
Hardness
resistance to indentation, scratching, abrasion, and wear
Toughness
ability to absorb energy and deform without fracturing (does not consider cracks)
Fatigue resistance
ability to resist crack growth and fracture when subjected to repeated cyclic loading
Corrosion resistance
a material's ability to withstand deteriorating/chemical breakdown in corrosive environments
Creep resistance
a material's ability to resist creep deformation
Creep deformation
very slow (almost imperceptible) deformation when material is subjected to a load for a longperiod of time (usually at elevated temperatures)
Elastic deformation
reversable deformation
elastic modulus and hooks law
Plastic deformation
irreversible post yield hardening law
Yield strength (𝝈𝒀)
the onset of plastic deformation• Defined as 0.2% offset stress: amount of stress required to create plastic strain of 0.002
Ultimate Strength (𝝈𝑼)
the highest engineering stress the material can withstand
Percent elongation
amount material lengthens before fracture
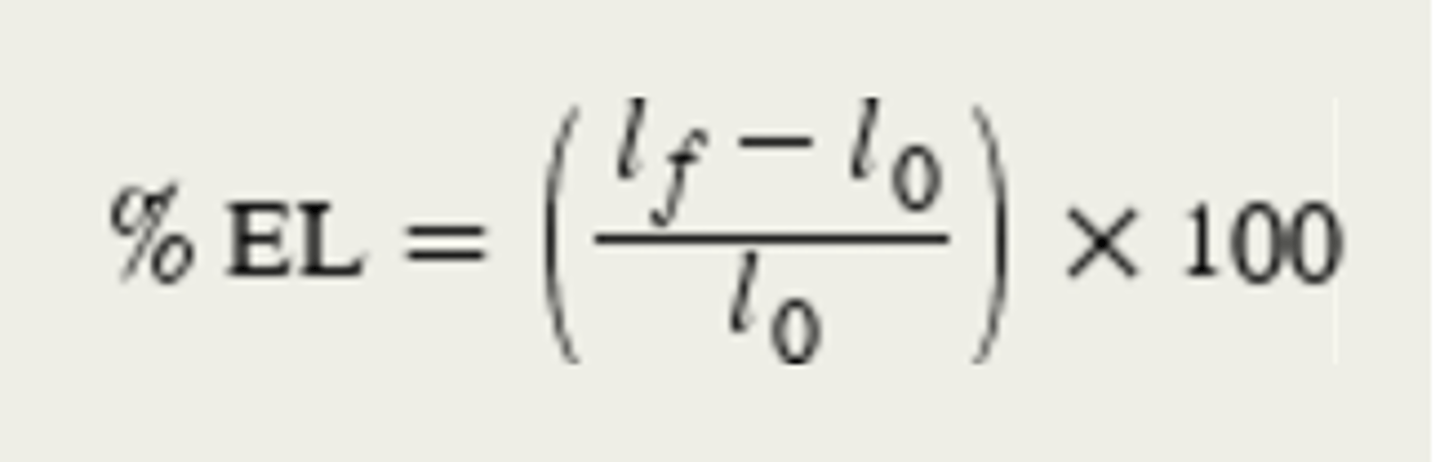
Percent reduction in area
amount the area is reduced before fracture

True stress
stress measure based on current cross-sectional area
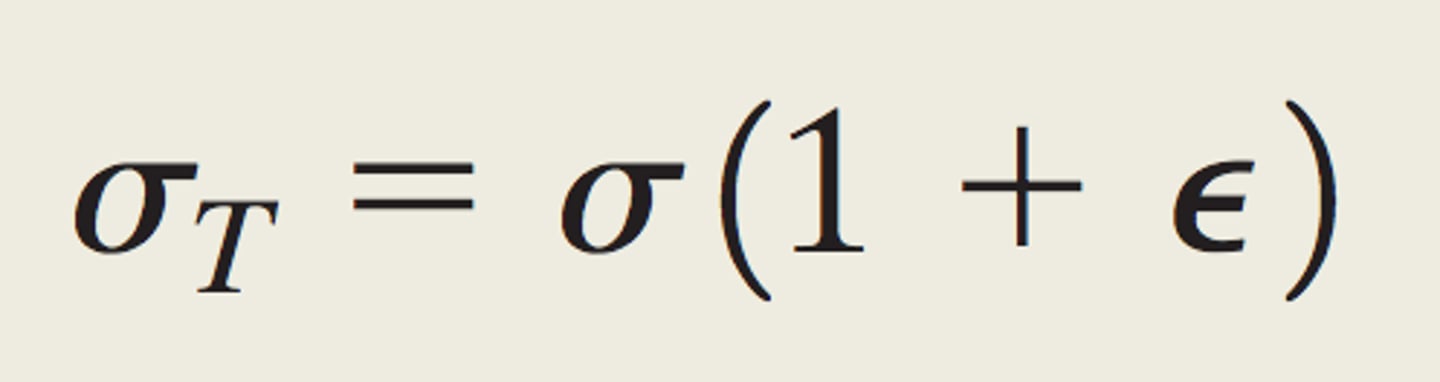
True strain
the elongation of the specimen in increments of instantaneous change in length
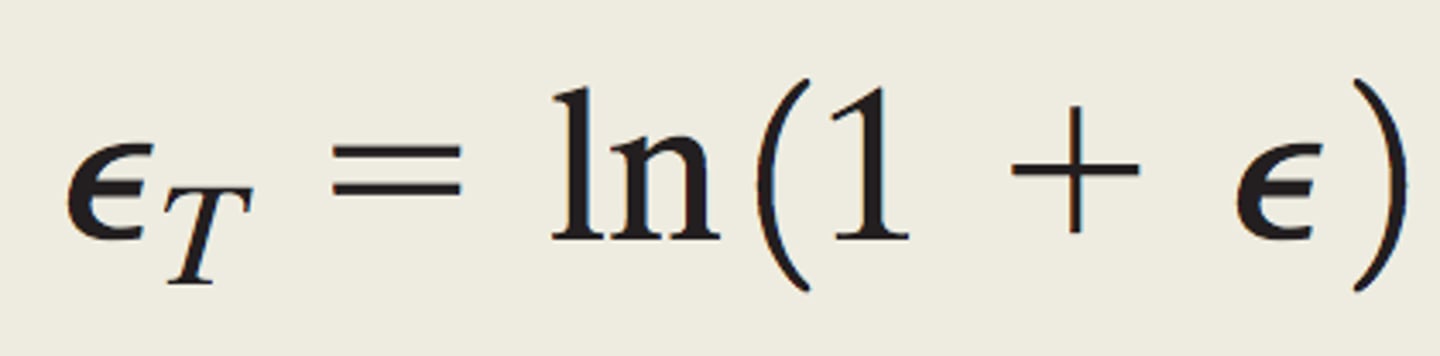
Work Hardening (Strain Hardening)
he increased yield/flow stress and strengthening of a material that occurs after plastic deformation
After each manufacturing step that plastically deforms material, increases the material strength and decreases the ductility
Flow Stress, 𝝈𝒇
amount of stress to continue deformingthe material (aka, "keep it flowing")
𝜎𝑓 will typically____?
increases as 𝜀 increases due to work hardening
Means that manufacturing operations require higher and higher forces to continue deforming the material
Power Law Hardening
K: strength coefficient
𝑛: strain hardening exponent
The true stress v. true strain post-yield hardening behavior often described
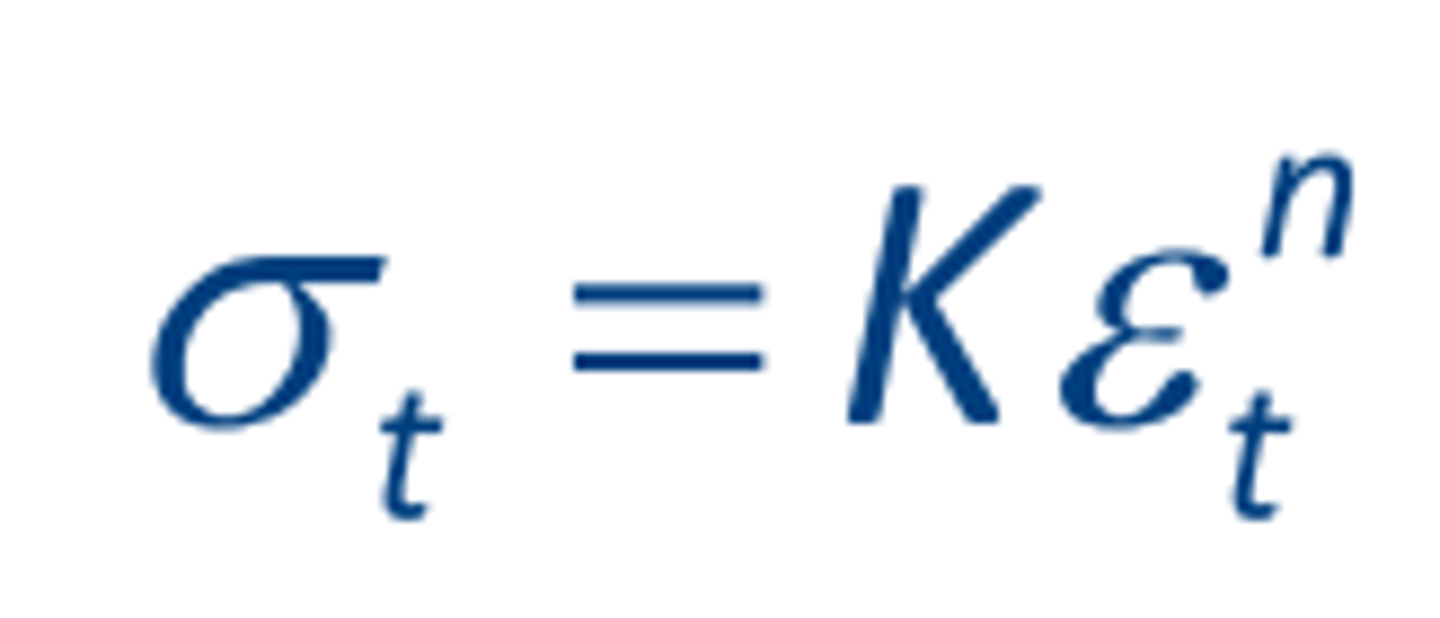
What is Hardness?
Hardness is associated with increased 𝐸 and 𝜎𝑌, which in harder materials cause increased abrasion and wear resistance of the material, and decreased ductility
Hardness testing?
is a two step process: load an indenter with known load,then measure the size of the indentation
Hardness testing is a quick___?
convenient, and non-destructive method of testing general material properties
hardness values
based on the difference in depths of indentation produced by a minor and major load
Toughness units
kJ/m^3
Toughness ?
Can be calculated by integrating stress-strain curve
•Often measured using a Charpy impact test
•Toughness is a function of both the material strength and ductility
fracture toughness (Kc)
ability of a material with a pre-existing crack to resist further crack growth and catastrophic fracture
fracture toughness (Kc) formula
𝑌: geometric scaling factor (assume 𝑌 = 1 for this class)
𝜎: applied stress
𝑎: crack length
• Edge cracks: physical crack length = a
• Internal cracks: physical crack length = 2a
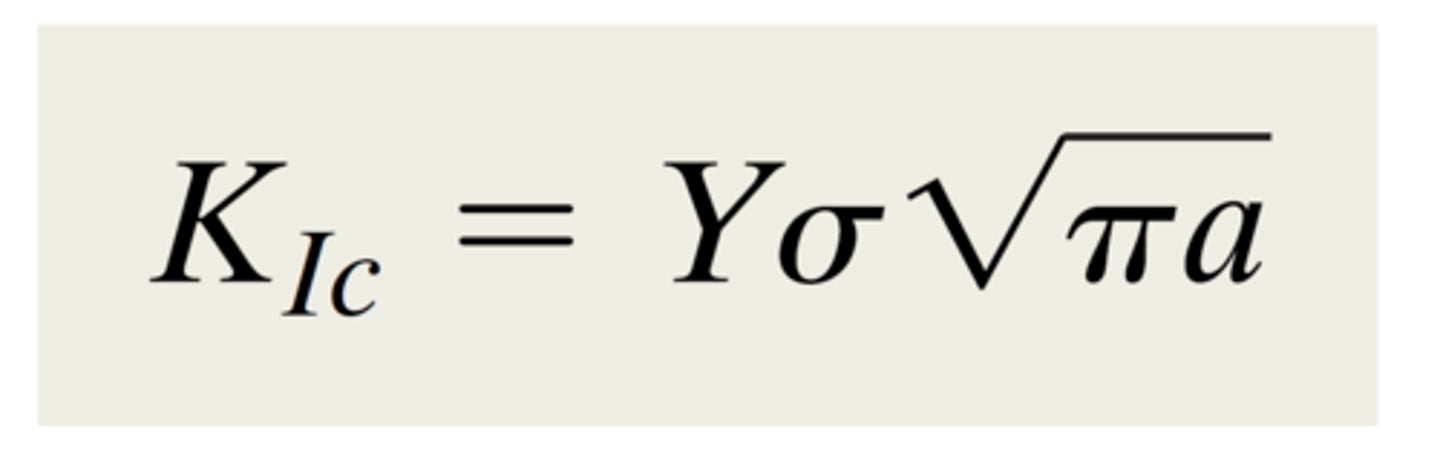
Fatigue
crack growth and material fracture caused by cyclic loading
Applied stresses are much lower than 𝜎𝑌 and 𝜎U
Yet fracture still eventually occurs due to gradual growth of cracks
Fatigue Lifetime
how many loadings cycles the material/structure can withstand before fracture occurs
∆𝜎 (Delta Sigma)
𝜎max−𝜎min
𝜎mean
(𝜎max+𝜎min)/2
S =?
𝜎a = (𝜎max-𝜎mean)/2
R
-(𝜎min/𝜎max)
Endurance Limit
the maximum cyclic stress amplitude (fully reversed loading) the material can withstand and never experience fatigue failure • Not all materials have one • Design to operate under this stress level to ensure durability over time.
S-N Curve
-plot of stress amplitude (S) vs. corresponding number of cycles to failure (N) for a material
-Allows for estimation and design decisions of the number of cycles until component failure at a given loading condition
S-N Curve formula
∆𝜎*(NF)^a = C
increasing the temperature will?
-Decrease strength
-Decrease elastic modulus
-Decrease endurance limit and fatigue strength
-Increase the ductility
What happens to metals at a high temperature?
They become softer more ductile
Lower temperature
Stiffer more brittle
Creep
permanent deformation under a static load that is below the typical 𝜎y if load is maintained for long periods at elevated temperatures
Creep in metal occurs
high-temperature
Creep occurs in polymers at more
moderate temperatures
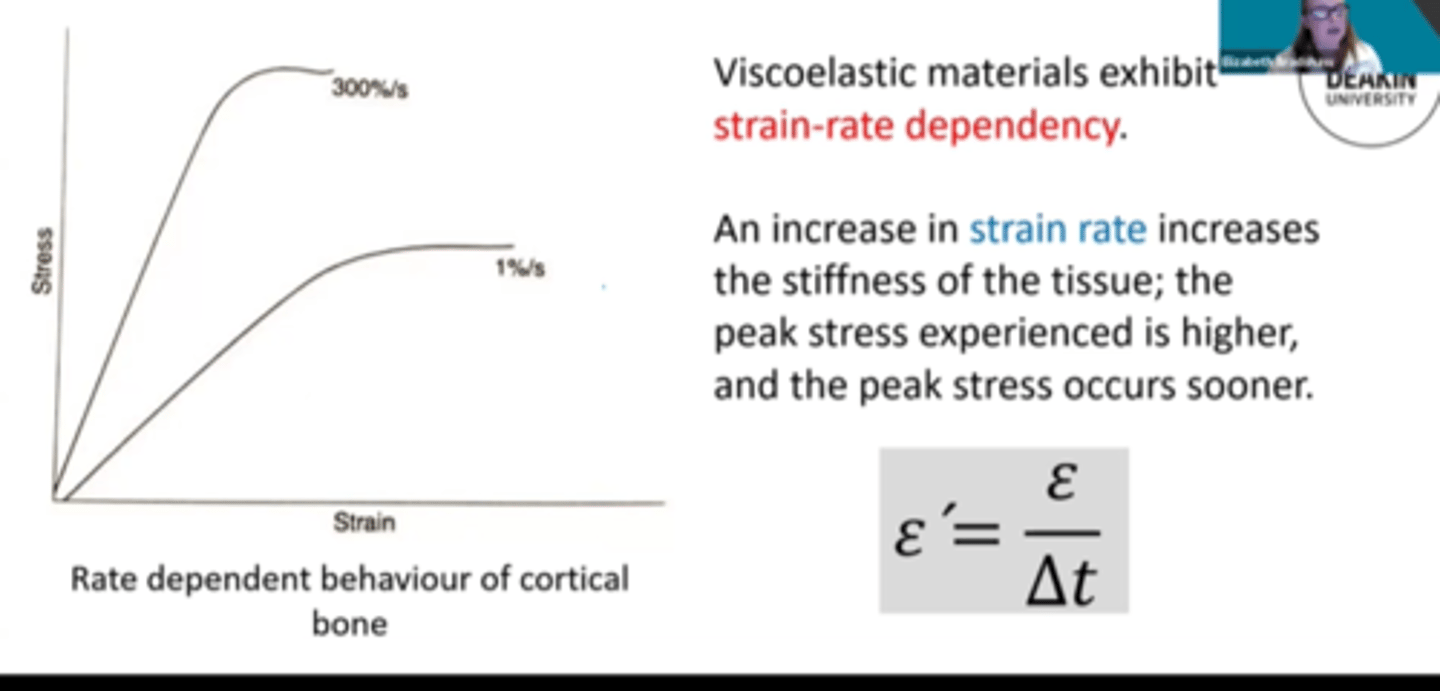
Strain Rate
the amount of deformation induced per unit time
Hertz is a measure of frequency?
not strain per unit time.
Increased strain rate generally causes
-Increase strength
-Decrease ductility
percent elongation
-No effect elastic modulus
Elevated temperatures generally cause
-decreased strength, increased ductility
Johnson-cook model?
𝐴,𝐵,𝑛,𝑐,𝑚: constants fitted to material behavior
𝜀: reference strain rate 𝑇𝑟: reference temperature
𝑇𝑚: melting temperature
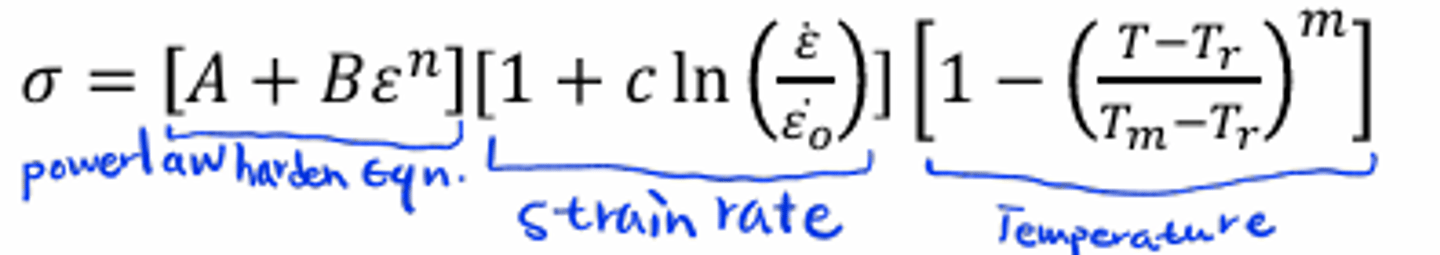
Ionic bonding
Electrons transferred
Metallic and non-metallic elements
Ionic bonding
Characteristics of this bonding type are,
Moderate to high strength
High hardness
Brittle
high melting point
Low conducive
Covalent bonding
Electrons are shared
non-metal to non-metal
covalent bonding characteristics
High strength
high melting point
brittle
Generally insulate
metalic bonding
Sharing a "sea" of electrons that are loosely held together and can travel through the metal
metallic bonding characteristics
Moderate to high strength
moderate to high melting point
high ductility
conductive
Difference between ceramics and polymers
Crystalline or amorphous arrangement difficult to deform
Polymers stretch and untangle because of the polymer chain
Allotropy
Of an element, having more than one form
carbon : diamond, graphite
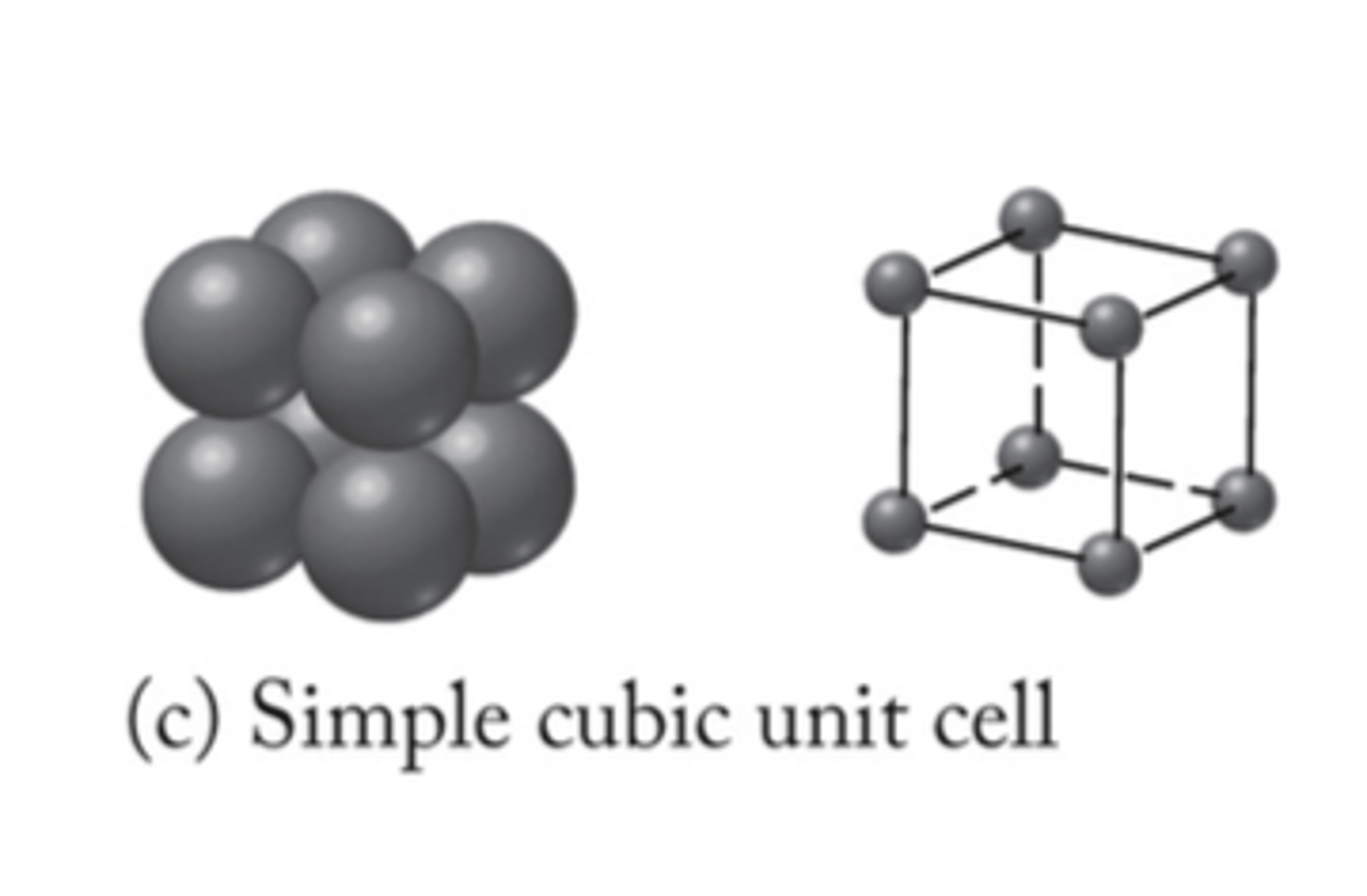
simple cubic unit cell
None in typical metals
lowest packing factor
52%
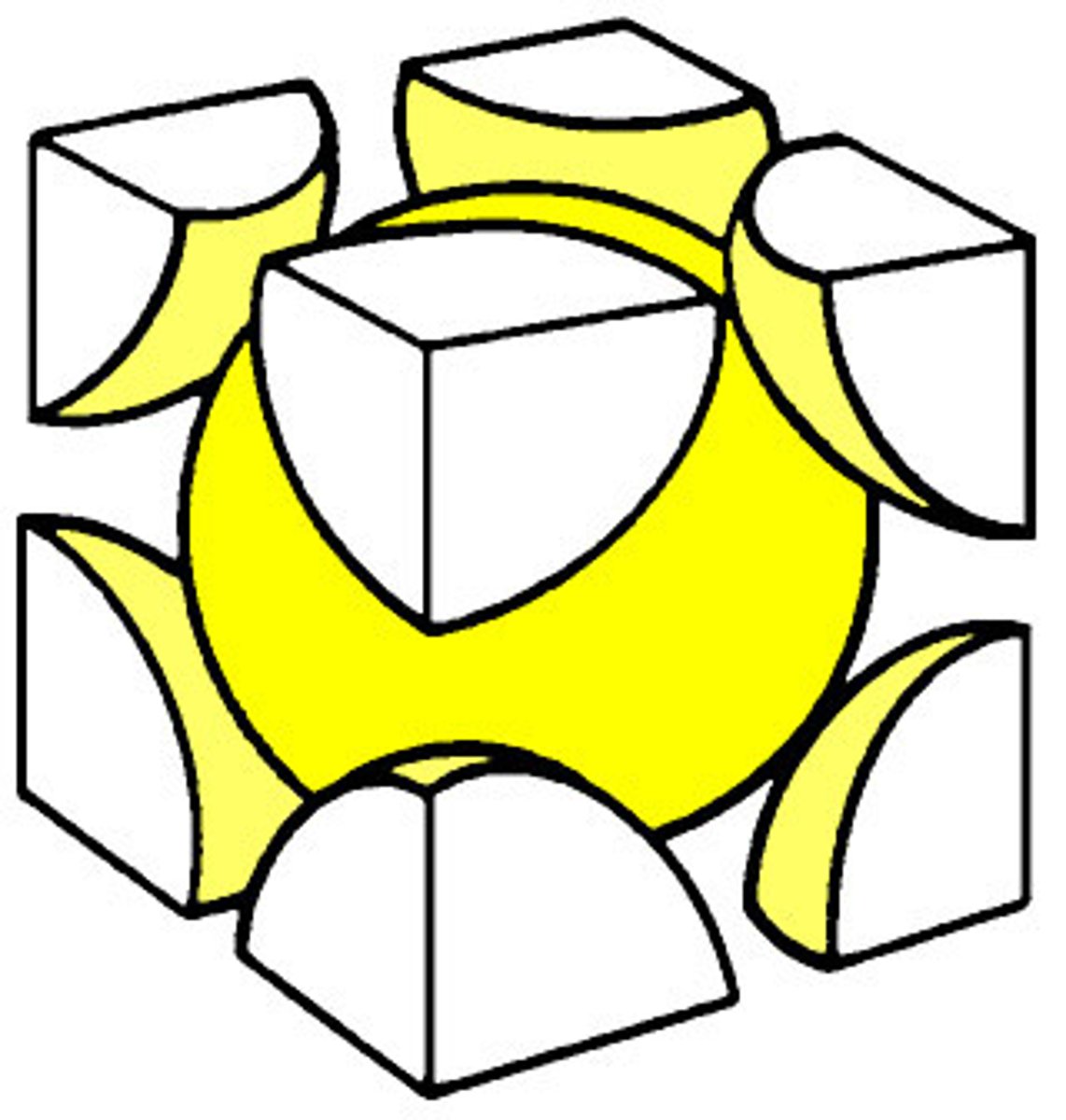
Body Centered Cubic (BCC)
Fe, Cr, Mn, W, Nb, V
68% packing factor
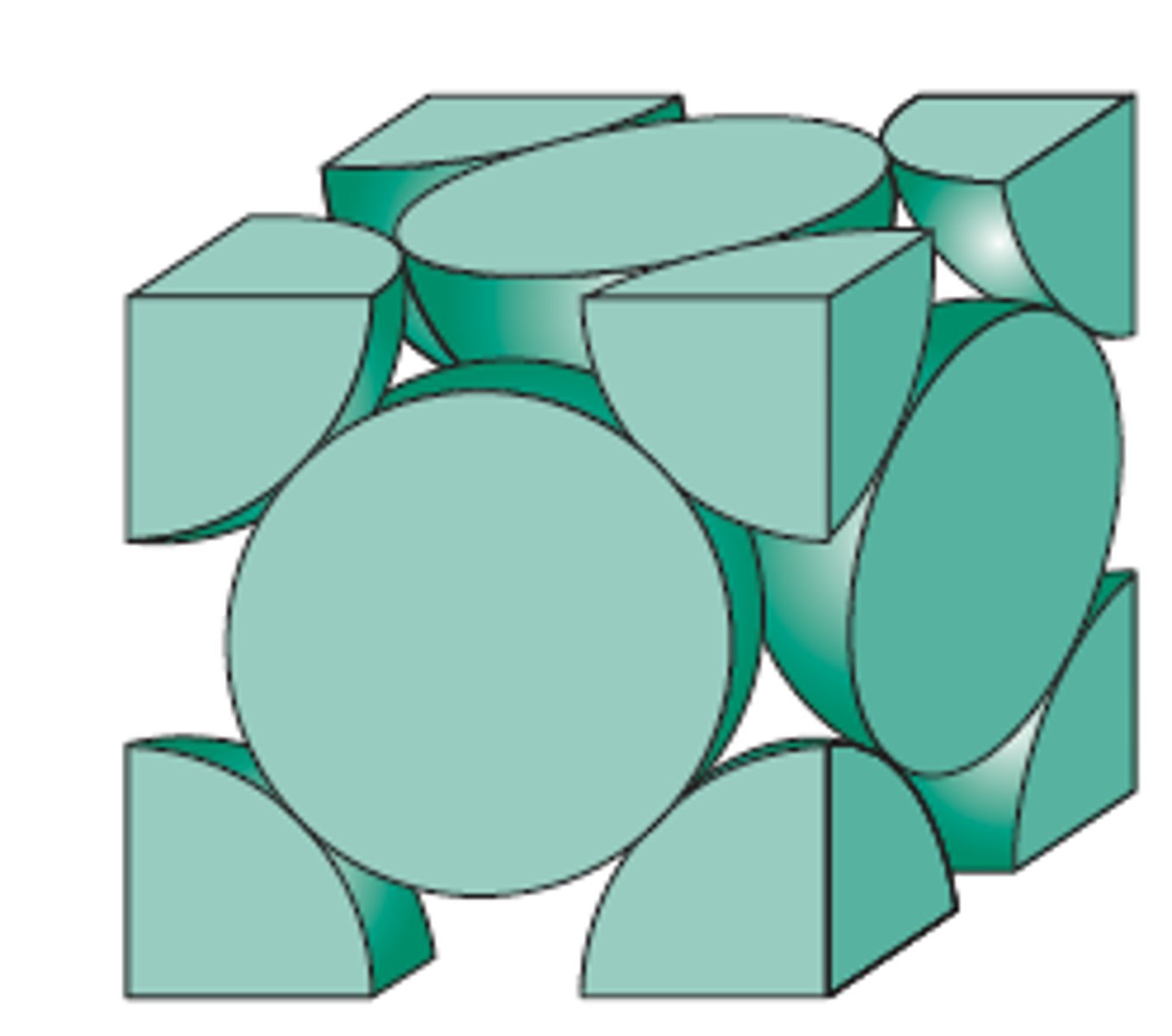
Face Centered Cubic (FCC)
Fe, Al, Cu, NI, Au
74% par=cking factor
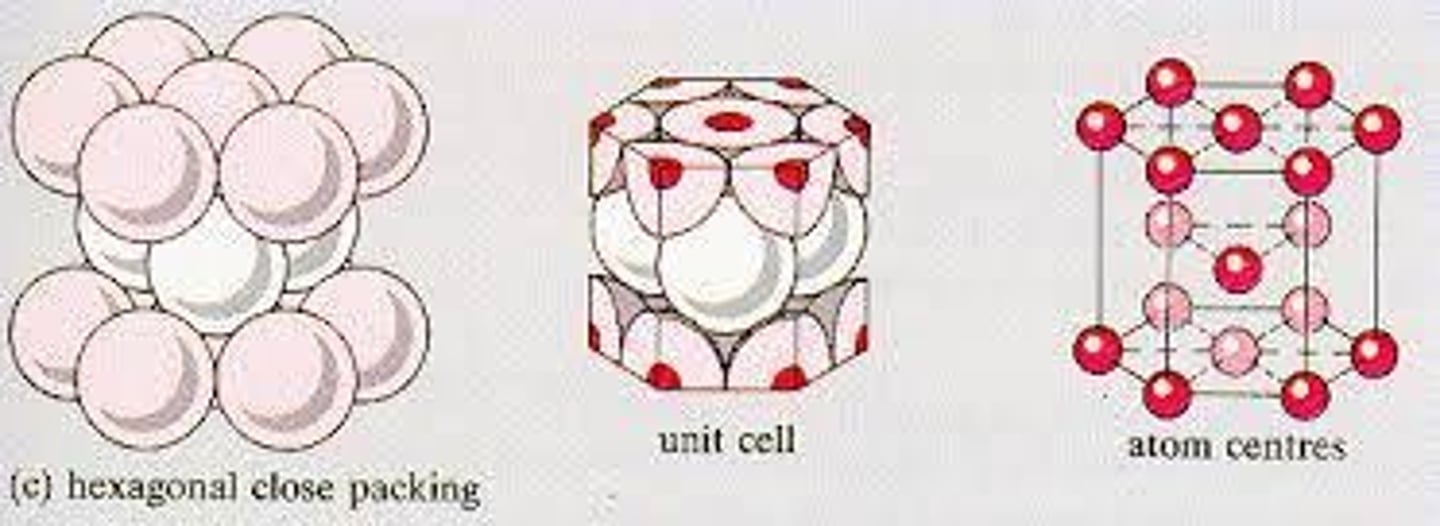
hexagonal close-packed (HCP)
Ti, Mg, An, Ar
74% packing factor
Single Crystals
crystalline materials consisting of a single crystal
only possible in small scale
Polycrystalline materials:
Have many grains, separated by grain boundaries
most material
Grain Nucleation
Particle form as metal cools
Grain Growth
More particles attach to the initial one, different orientations
As grains grow large enough, they encounter other _________ which obstructs the__________ growth
grain,
Upon complete solidification, grains with___________formed
irregular shapes and multiple orientations
grain boundaries
Surfaces that divide the grains and form boundaries between them
Defects in Crystal Structure
Grain boundaries
Point defects
dislocations
intrinsic defects
Vacancy atoms
interstitial atoms
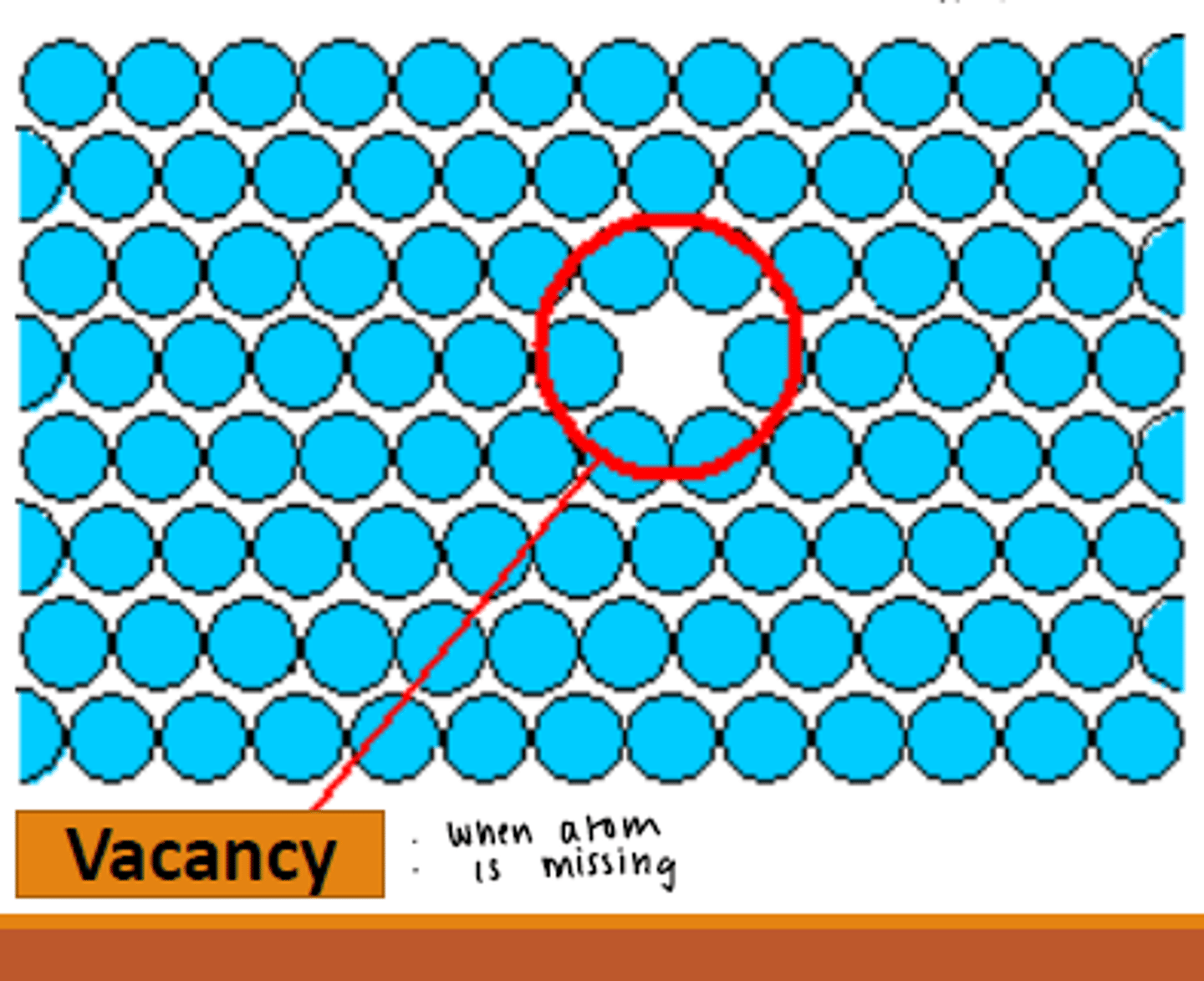
vacancy
Suppose be an atom there but no
Interstitial atom
extra atom in the lattice
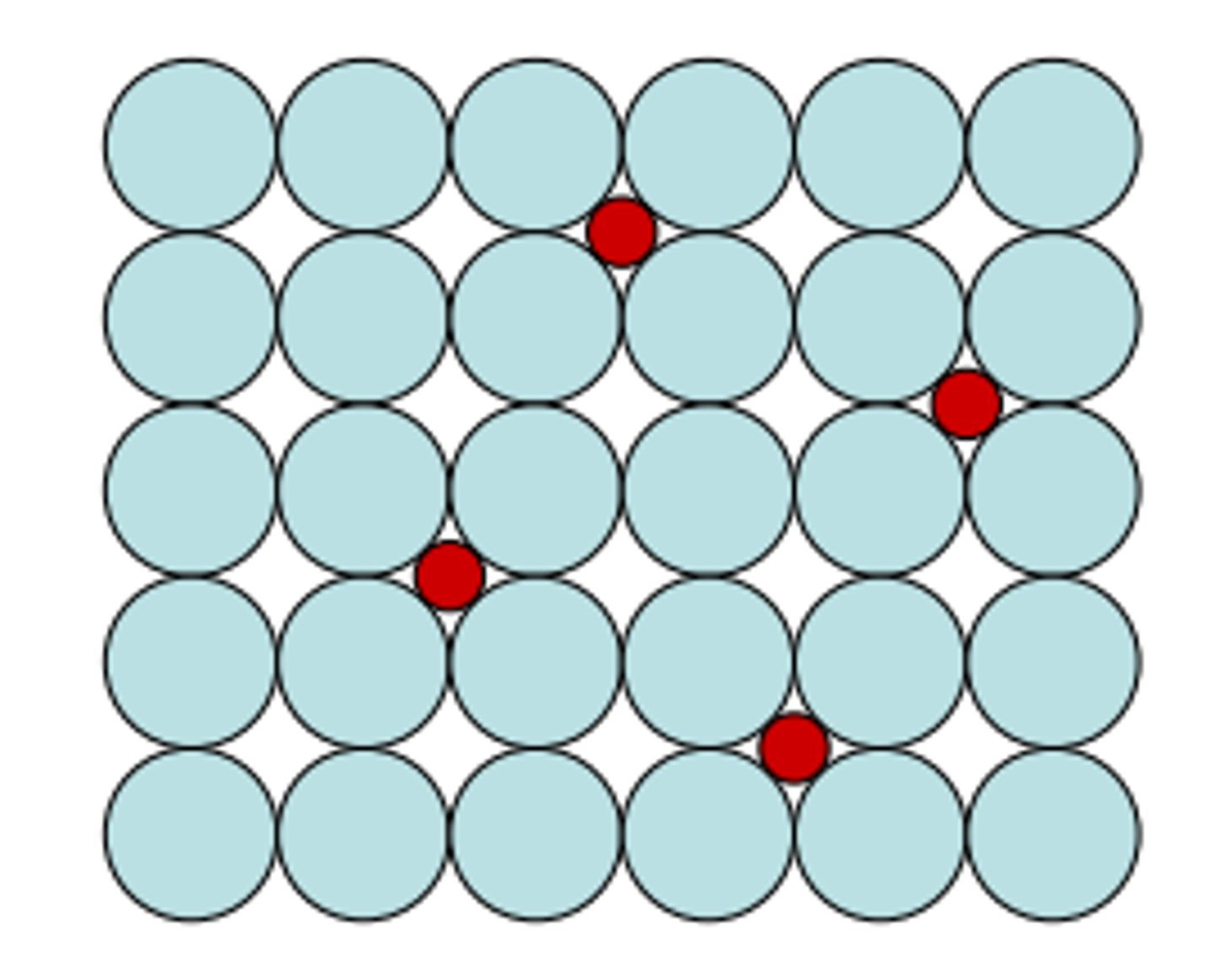
Solutes atoms
Intentionally added
Solid Solution Strengthening
Adding atom to make metal strong
Impurities
Unintentionally have
Dislocations
Half of row of atoms are missing
Dislocations effects on materials
Make metals weaker
Increase ductility
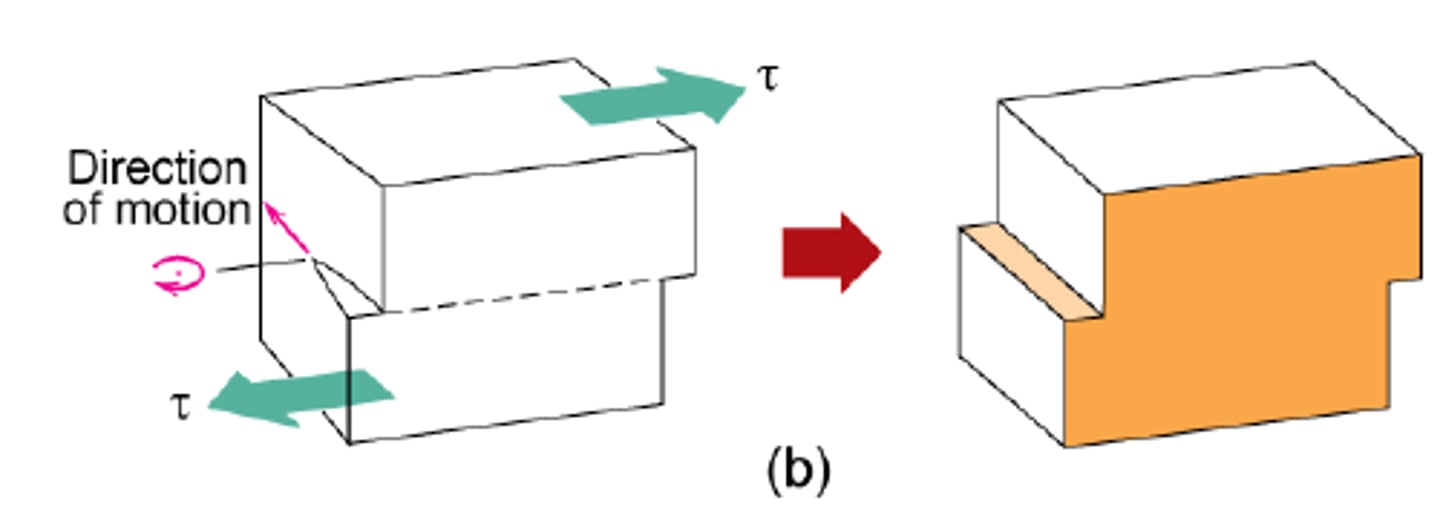
Slip
dislocations move under stress
plastic deformation
Slip direction
direction of dislocation movement
most densely packed line with atoms
Slip plane
plane which dislocation motion occurs
most densely packed plane with atoms
slip systems
the possible ways a dislocation can move
combination of slip plane and slip direction
Dislocation motion and slip
controls the plastic deformation of crystalline solids
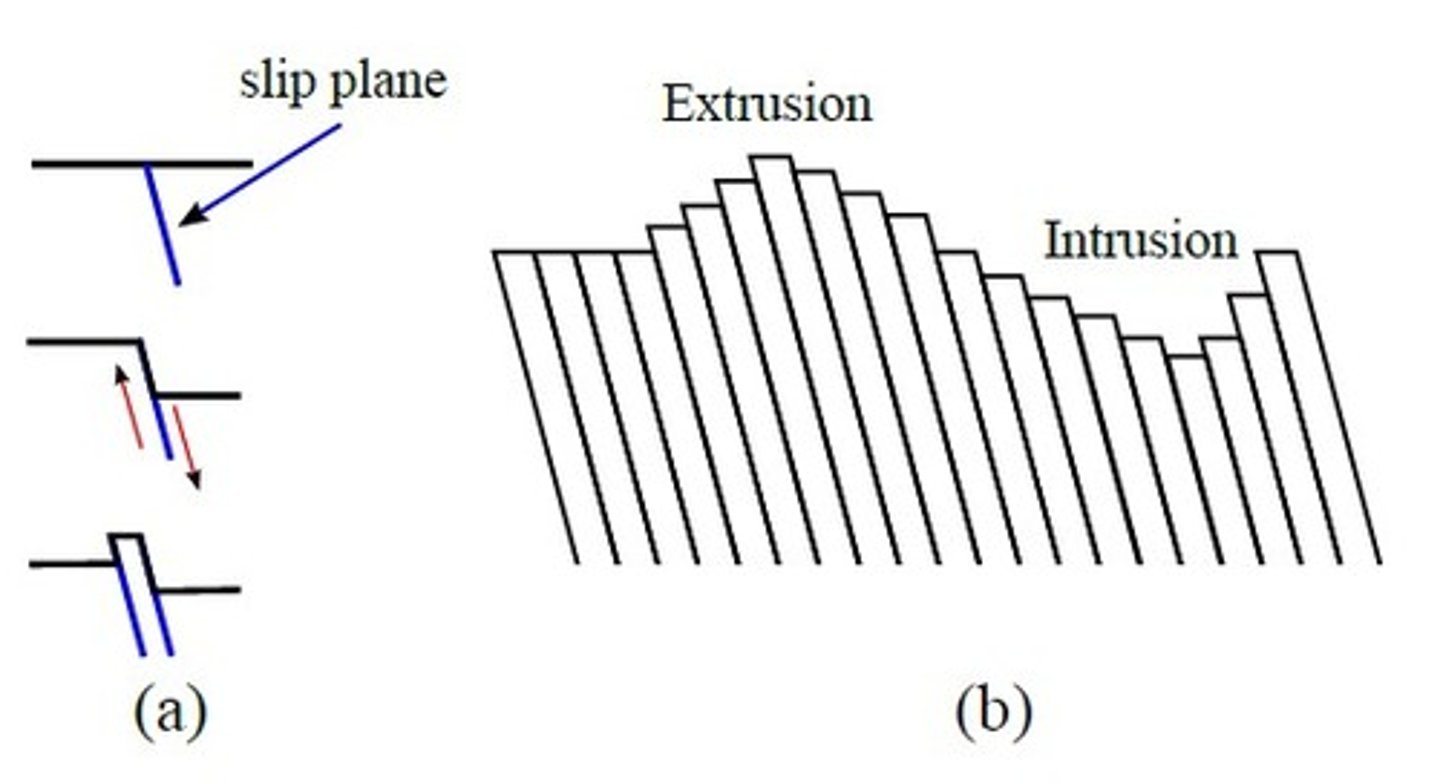
Slip bands
slip occur in slip systems makes bands
Edge dislocation
line moves parallel to the allied shear direction
Screw dislocation
perpendicular to the applied shear direction
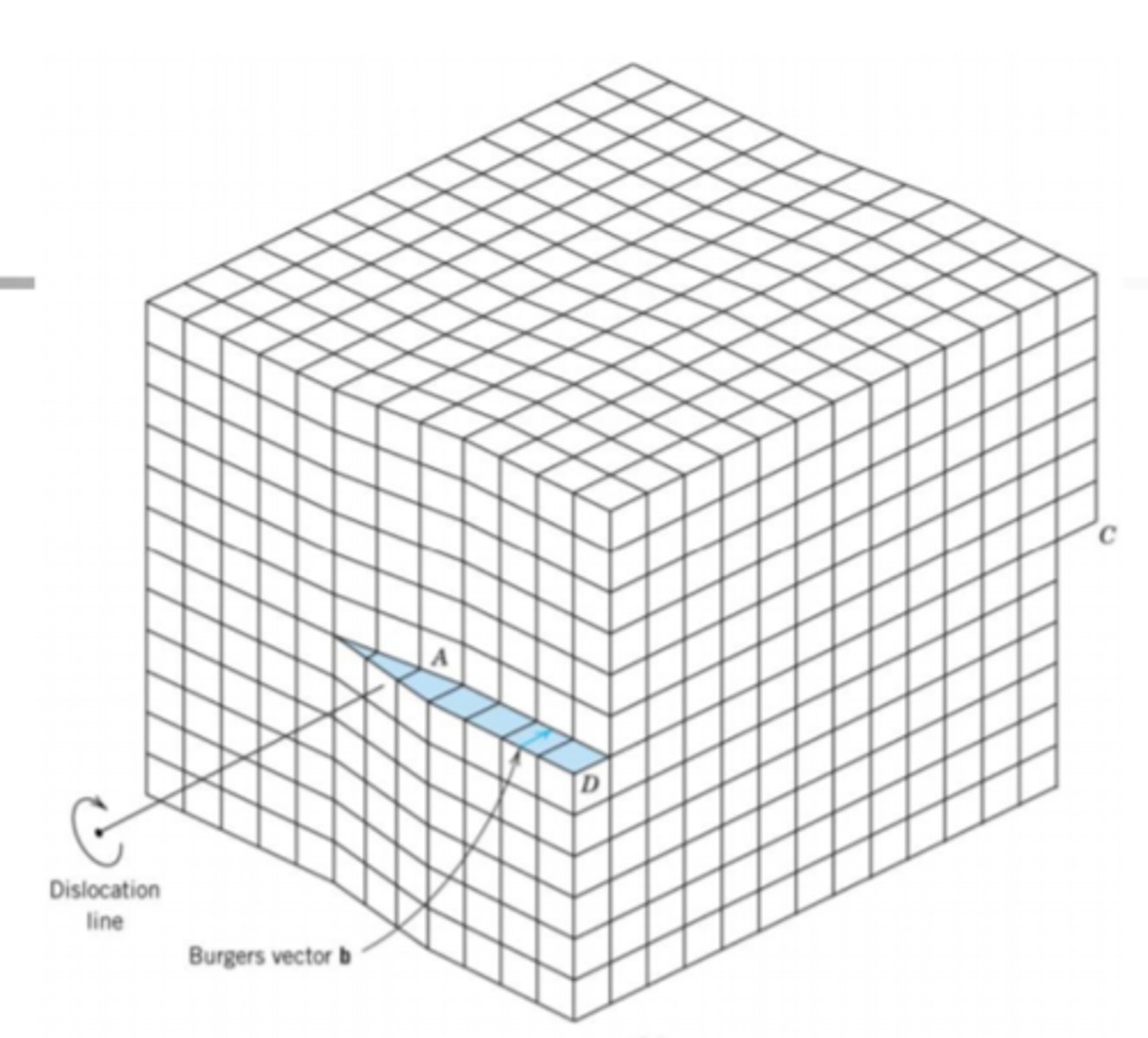
Dislocations in Metals
easy breaking/re-forming of bonds to move dislocation
easy to deform and have high ductility
Dislocations in ceramics
do not allow easy dislocation movement
brittle hard