AP Biology Unit 3: Cellular Energetics
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
1st Law of Thermodynamics
Energy can be transferred or transformed, but not created or destroyed. Ex. energy used by living things is recycled
2nd Law of Thermodynamics
Every energy transfer or transformation increases the entropy of the universe.
Metabolism
The totality of an organism's chemical reactions, consisting of catabolic and anabolic pathways, which manage the material and energy resources of the organism.
Metabolic Pathway
a specific molecule is altered in a series of steps to produce a product. Each step is catalyzed by an enzyme, macromolecules that speed up reaction
Catabolic Pathway
Releases energy by breaking down complex molecules into simpler compounds
Anabolic Pathway
consume energy to build complicated molecules from simpler ones
Energy
the capacity to cause change, can be used to do work-move matter against opposing forces such as gravity and friction
Kinetic Energy
energy of motion
Thermal Energy
Kinetic energy associated with random movement of atoms or molecules. Heat is the transfer of thermal energy
Potential Energy
energy that matter possesses because of its location or structure
Chemical Energy
A form of potential energy that is stored in chemical bonds between atoms.
Spontaneous v. Nonspontaneous Processes
occur without energy input; they can happen quickly or slowly. Can increase entropy while nonspontaneous ones decrease entropy and require input energy
Free Energy
Measures the portion of a system's energy that can perform work when temperature and pressure are uniform throughout the system, as in a living cell. Also the measure of a system's stability (Higher G--> more instability).If change in free energy/ G is negative it is spontaneous if it is 0 or positive it is nonspontaneous
Free Energy and Temparature
Change in free energy during reaction is reacted to temperature and changes in enthalpy (total energy) and entropy
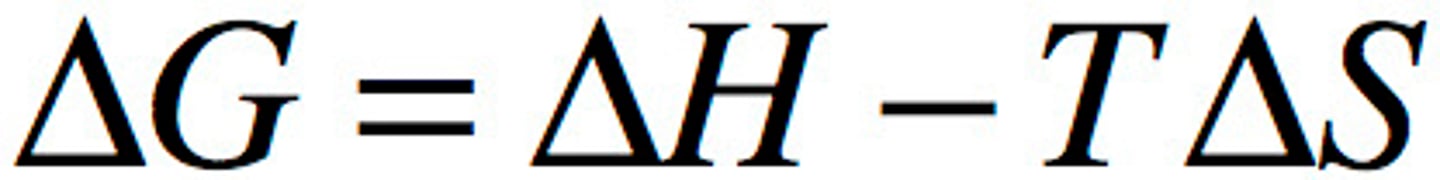
What happens if the reaction has a negative change in G?
The system loses free energy and becomes more stable
Exergonic Reaction
proceeds with a net release of free energy and is spontaneous. Products store less free energy than reactants
Endergonic Reaction
absorbs free energy from surroundings. Products store more free energy than reactants
Energy Coupling
The use of an exergonic process to drive an endergonic one.
ATP
Adenine Tri-Phosphate; composed of ribose, adenine, and 3 phosphate groups; mediates energy coupling; releases energy when turns into ADP after a phosphate group is broken off by hydrolysis
Catalyst
A chemical agent that speeds up a reaction without being consumed by the reaction.
Enzyme
A macromolecule, usually a protein, that serves as a biological catalyst, changing the rate of a chemical reaction without being consumed by the reaction.
Activation Energy
the minimum amount of energy required to start a chemical reaction. Heat often supplies this energy.
Catalysis
A process by which a chemical agent called a catalyst selectively increases the rate of a reaction without being consumed by the reaction. Enzymes do this by lowering the activation energy barrier enough for the reaction to occur at a moderate temperature
Substate
reactant of an enzyme-catalyzed reaction
Enzyme-substrate complex
A temporary complex formed when an enzyme binds to its substrate molecule(s).
Active Site
a region on an enzyme that binds to a protein or other substance during a reaction.
Induced Fit
The change in shape of the active site of an enzyme so that it binds more snugly to the substrate, induced by entry of the substrate.
Effect of Temperature on Enzymes
Each enzyme has an optimal temperature where the reaction rate is at its maximum potential. Past this temperature denaturation occurs
Effect of pH on Enzymes
each enzyme has optimal pH, pH can denature enzymes but this is unusual in a living thing
Cofactors
nonprotein enzyme helpers
Covalently Bonded Inhibitors
Aren't reversible
Competitive Inhibitors
closely resemble the substrate, and can bind to the enzyme's active site blocking the substrate form binding. This can be fixed by an increase in substrate concentration
Noncompetitve Inhibitors
bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective
Allosteric Regulation
The binding of a regulatory molecule to a protein at one site that affects the function of the protein at a different site.
Feedback Inhibition
A method of metabolic control in which the end product of a metabolic pathway acts as an inhibitor of an enzyme within that pathway.
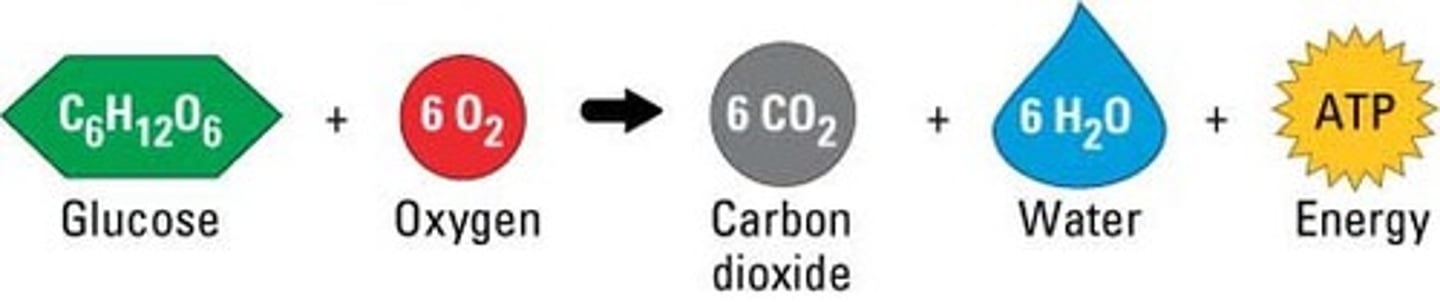
Cellular Respiration
Process that releases energy by breaking down glucose and other food molecules in the presence of oxygen
Redox Reactions
Chemical reactions that transfer electrons between reactants. Loss of electrons is called oxidation whereas addition of electrons equal to reduction. The electron donor is called the reducing agent and the electron acceptor is called the oxidizing agent
Stepwise Energy Harvest
In cellular respiration, glucose and other organic molecules are oxidized in several steps. These electrons that are extracted do not travel alone, they often travel coupled with a singular proton, and since 1 proton combined with 1 electron results in the creation of a Hydrogen atom we can simply say that electrons travel as Hydrogen Atoms. However, electron carriers like NAD+, a coenzyme that functions as a electron carrier and oxidizing agent, can also take up these electrons, and once it takes in an electron it turns into NADH
Electron Transport Chain
the series of molecules built into the inner membrane of the mitochondria. NADH passes electrons to the ETC where they are transferred in a series of redox reaction, each receiving a small amount of energy. Oxygen is the final electron acceptor and captures the electrons and H+ forming H2O, energy yielded used to make ATP
Glycolysis
Occurs in the cytoplasm and has 2 main phases:
1.) Energy investment phase: 2 ATP used phosphorylates to 2 3-carbon sugar molecules or G3P (no oxidation occurs in this phase!!)
2.) Energy payoff phase: 4 ATP synthesized (by enzymes working in this phase that phosphorylate ADP), 2 NAD+ reduced to NADH (one for each G3P), the small sugars are oxidized to make 2 pyruvate and 2 water.
Net 2 ATP made in glycolysis
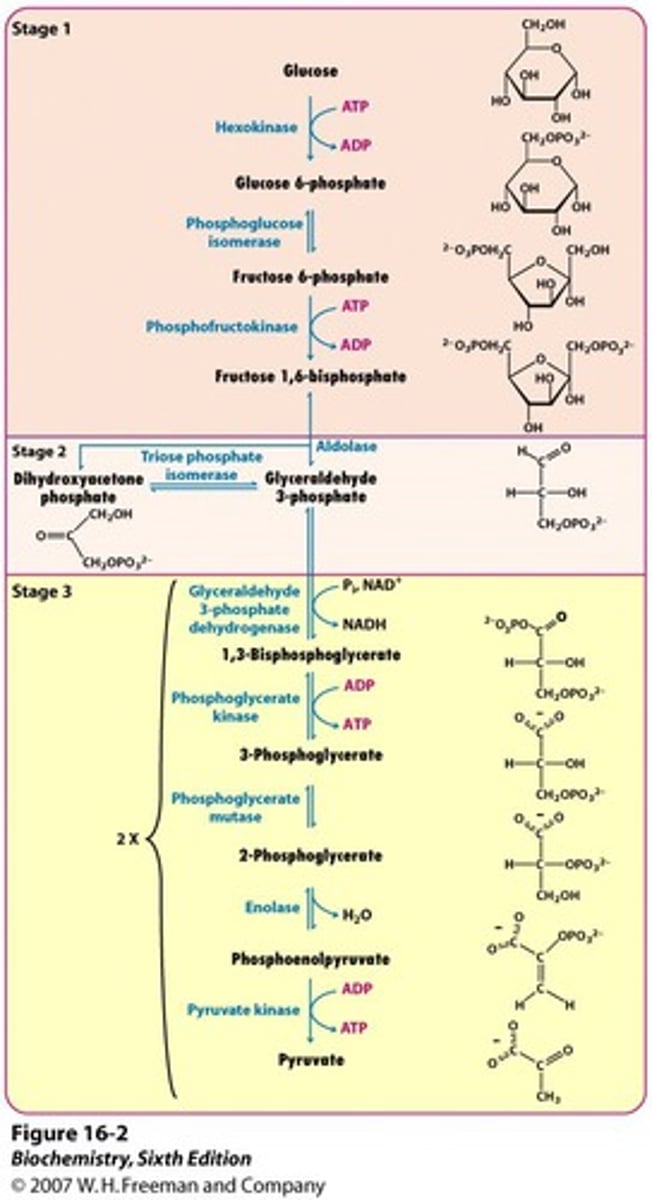
Oxidation of Pyruvate to Acetyl CoA
Pyruvate dehydrogenase catalyzes three reactions
1. Oxidation of pyruvate's carboxyl group, releasing
the first CO2 of cellular respiration
2. Reduction of NAD+ to NADH
3. Combination of the remaining two-carbon fragment with coenzyme A to form acetyl CoA
Citric Acid Cycle
1.) Acetyl CoA adds a 2-carbon acetyl group to oxaloacetate making citrate
2.) Citrate converted to its isomer, isocitrate, by removal of water molecule and addition of another
3.) Isocitrate oxidized, reducing NAD+ to NADH. The resulting compound loses a CO2 molecules
4.) CO2 lost again and the resulting compound is oxidized, reducing NAD+ to NADH. Remaining molecule attacked to CoA by unstable bond
5.) CoA displaced by a phosphate group, which is transferred to GDP making GTP used to generate ATP
6.) 2 hydrogen transferred to FAD making FADH2 and oxidizing succinate
7.) Addition of water rearranges bonds is substrate
8.) Substrate oxidized reducing a NAD+ to NADH and regenerating oxalocacetate
-Makes 1 ATP, 3 NADH, 1 FADH2, and 2 CO2 as waste
oxidative phosphorylation
The electron transport chain and chemiosmosis facilitate synthesis of most cells of ATP. NADH and FADH2 account for most of the energy extracted from glucose. They donate electrons to the etc which powers ATP synthesis via oxidative phosphorylation.
Electron Transport Chain (Cellular Respiration)
NADH and FADH2 donate electrons to different electron acceptors early in the chain. Electrons are passed through a number of carrier molecules including cytochromes. Electrons drop in free energy as they are transferred to the chain
Chemiosmosis (oxidative phosphorylation)
Energy released as electrons are passed down the etc is used to pump H+ from the mitochondrial matrix to intermembrane space. H+ then moves down the concentration gradient back across the membrane, passing through the ATP synthase. H+ binds into binding sites on the rotor of ATP synthase, causing it to spin in a way that catalyzes phosphorylation of ADP to ATP. Certain electron carriers in the ETC accept and release H+ along with the electrons
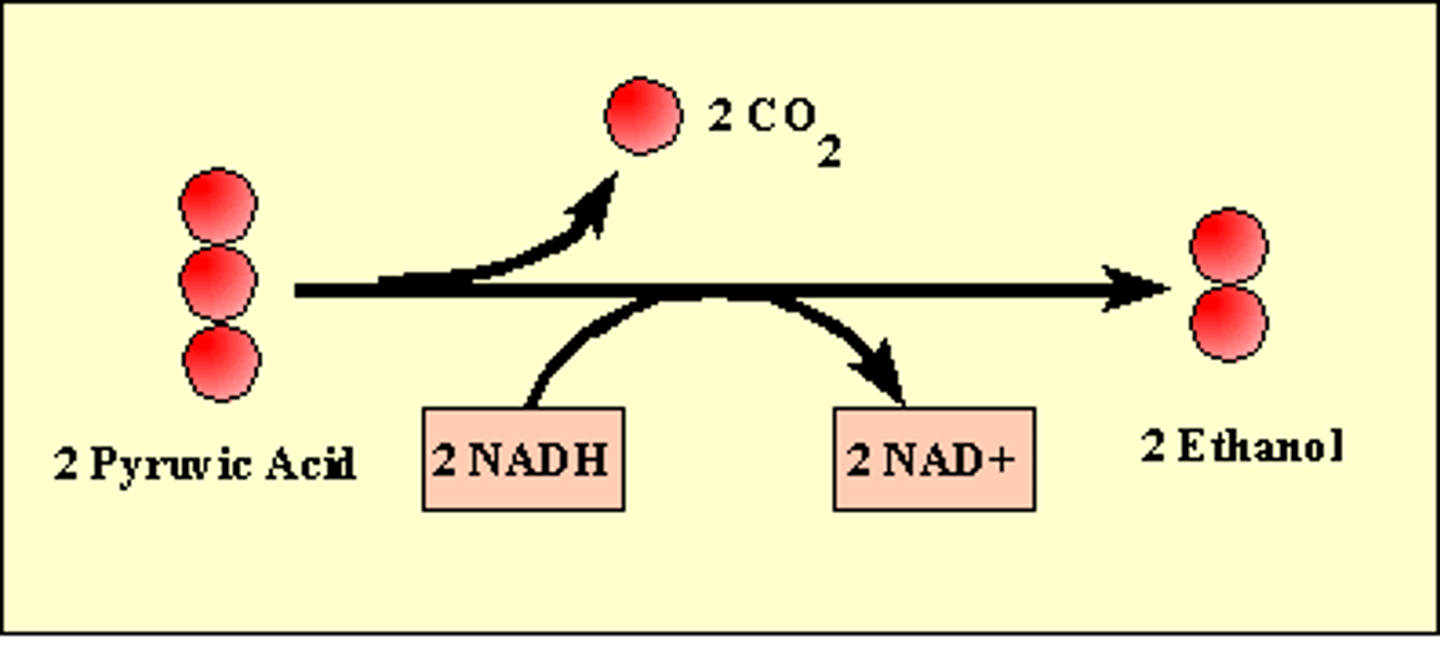
Alcohol Fermentation
Glycolysis followed by the reduction of pyruvate to ethyl alcohol, regenerating NAD+ and releasing carbon dioxide.
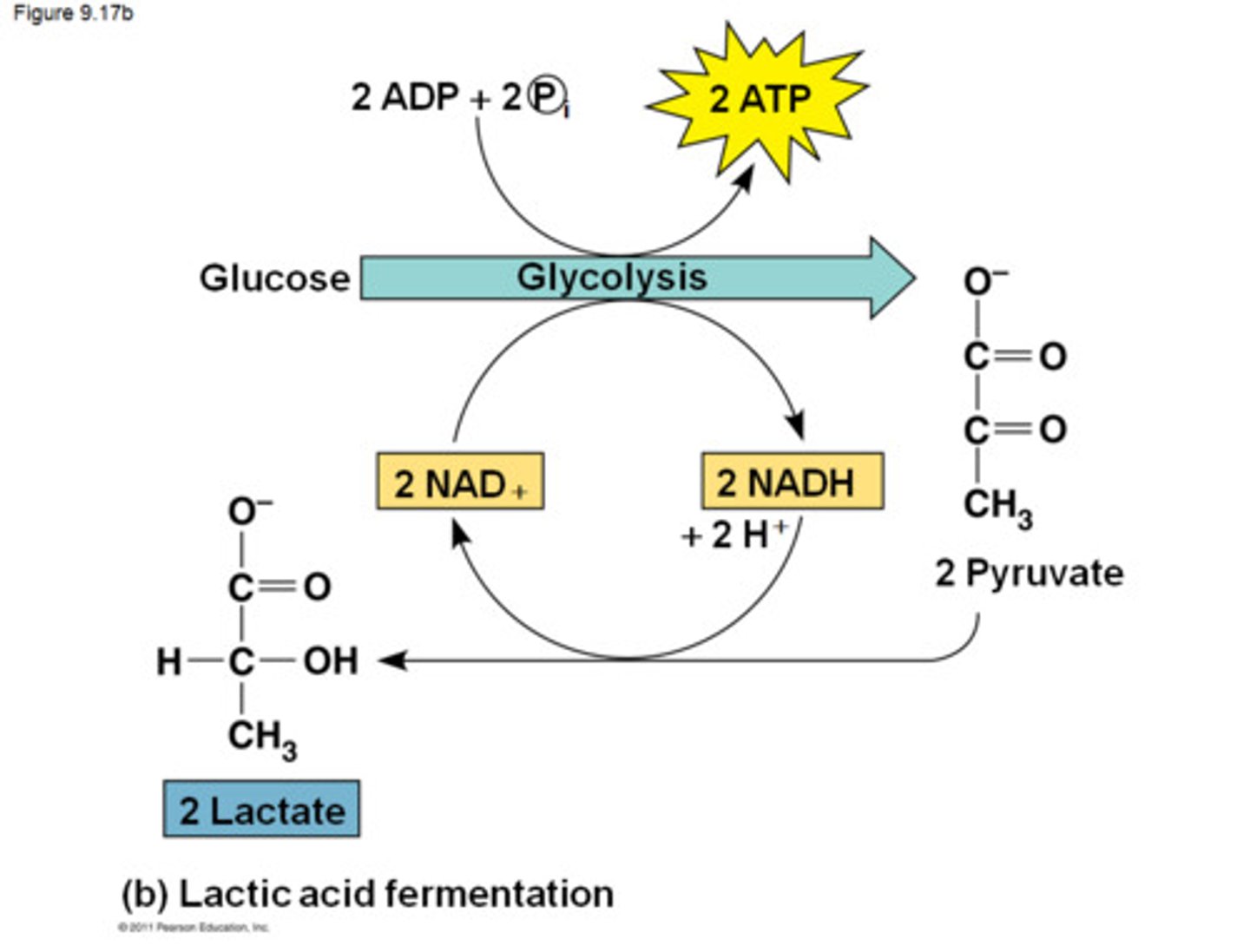
Lactic Acid Fermentation
the chemical breakdown of carbohydrates that produces lactic acid as the main end product
Photosynthesis
The process that converts solar energy into chemical energy within chloroplasts
Chloroplasts
In plants and other photosynthetic organisms; allow for chemical reactions of photosynthesis ; most photosynthesis occurs in the leaves and chloroplasts are found in the mesophyll, interior tissue of leaves. CO2 enters and O2 exist via stomata, microscopic pores in leaves.
Splitting of Water
Chloroplasts split H2O into hydrogen and oxygen, incorporating the electrons of hydrogen into sugar molecules and releasing oxygen as a by-product
Photosynthesis as a Redox Process
Photosynthesis reverses the direction of electron flow compared to respiration
Photosynthesis is a redox process in which H2O is oxidized and CO2 is reduced
Photosynthesis is an endergonic process; the energy boost is provided by light
Photons
light behaves as though it consists of discrete particles; each photon has a fixed quantity of energy
Chloropyll A
the key capturing pigment that participates directly in light reactions
Photosystem
A reaction-center complex surrounded by light-harvesting complexes
Reaction-Center Complex
An association of proteins holding a special pair of chloropyll a molecules and a primary electron acceptor
Light-Harvesting Complex
Various pigments bound to proteins that transfer energy of photons to the chlorophyll a molecules in the reaction center-complex. The chlorophyll a molecules special because transfer an excite to a different molecule
Primary Electron Acceptor
Accepts excited electrons and is reduced by them
PSII (P680)
Reaction-center chlorophyll best at absorbing 680 nm light wavelength
PS I (P700)
Reaction-center chlorophyll best at absorbing 700 nm light wavelength
Linear Electron Flow
1.) photon hits a pigment in a light harvesting complex of PSII and its energy passed amount pigment molecules until it hits P680
2.) Excited electron form P680 transferred to primary electron acceptor; refer to oxidized form as P680+
3.) An enzyme catalyzes split of H2O into 2 electrons, to hydrogen ions, and oxygen
-Electrons transferred to P680+ pair, bringing back to P680
-Hydrogen ion released into thylakoid space
-Oxygen atom combines with another one by 2 different water molecules to make O2
4.) Electrons passed in a series of redox reaction from primary electron acceptor of PSII down an ETC to PSI
- The etc includes electron carrier plastoquinone (Pq), cytochrome complex, and a protein called plastocyanin (Pc)
-energy released in electron transfer used to pump hydrogen ions to thylakoid space, creating a proton gradient across thylakoid membrane
5.) Potential energy stored in proton gradient drive making of ATP in chemiosmosis
6.) In PSI, transferred light-energy excites P700, which loses electrons to primary electron acceptor
- P700+ accepts an electron passed down from PSII via the etc
7.) Electrons passed from primary electron acceptor of PSI down a second ETC to protein ferrodoxin (Fd)
- no proton gradient of ATP produced by this ETC
8.) The enzyme NADP+ reductase catalyzes the transfer of electrons from Fd to NADP+
- 2 electrons are needed to reduce NADP+ to NADPH
- electrons of NADPH are at a higher energy level than they were in H2O, so are more readily available for the reactions of the Calvin Cycle
- Formation of NADPH also removes a hydrogen ion form the stroma
Cyclic Electron Flow
photoexcited electrons cycle back from Fd to the cytochrome complex instead of being transferred to NADP+.
Chemiosmosis in Chloroplasts vs. Mitochondria
both generate ATP, but mitos transfer chemical energy from food to ATP, whereas chloroplasts transform light energy into chemical energy in ATP; both function because of strong pH (H+ concentration) gradients
The Calvin Cycle
-Cycle must take place 3 times, fixing 3 molecules of CO2, one for each turn of the cycle
1.) Carbon Fixation: the binding of CO2 to a 5 carbon sugar, ribulose biphosphate (RuBP) is catalyzed by RuBP carboxylase-oxygenase or rubisco; the 6 carbon intermediate molecule is immediately split into 2 molecule of 3-phosphoglycerate (for each CO2 fixed)
2.) Reduction: Each molecule of 3-phosphoglycerate is altered through phosphorylation by 6 ATP and reduction by 6 NADPH to ultimately produce G3P sugar; 4 every 3 CO2 molecule that enters the cycle, 6 molecule of G3P form
3.) Regeneration of CO2 Acceptor (RuBP): The remaining 5 molecule of G3P rearranges into a complex series of reaction yielding 3 molecules of RuBP ; 3 additional molecules of ATP are used to facilitate regeneration of RuBP