Chem unti 1 quiz 2
1/23
Earn XP
Description and Tags
Classification of Matter Separation of Mixtures Physical & Chemical Changes & Properties States of Matter
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Pure Substance
uniform & definite composition, cannot be separated by physical means
Element
cannot be broken down by chemical means, found on PT, 1 type of atom only
Compound
2 or more different elements chemically bonded in a fixed proportion, can be separated chemically into elements
Diatomic
any substance made up of only 2 atoms
Mixture
Physical blend of 2 or more substances, can be separated by physical means, proportions can vary

Homogeneous Mixture
uniform throughout, also known as solution, gases will be homogeneous

Heterogeneous Mixture
non-uniform throughout e.g sand & water
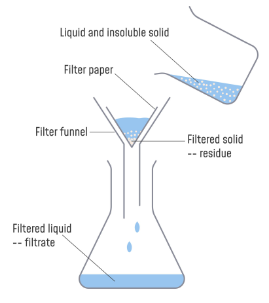
Filtration
Mixture of solid & liquid is poured through porous medium - liquid passes through while solid remains
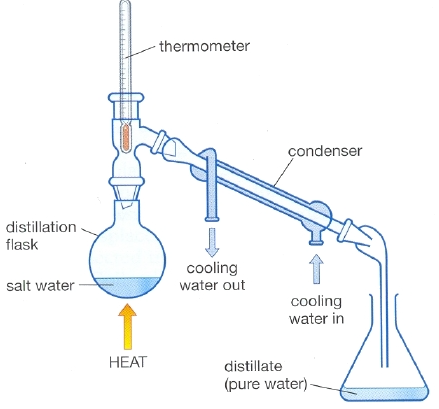
Distillation
separation of substances based on boiling point
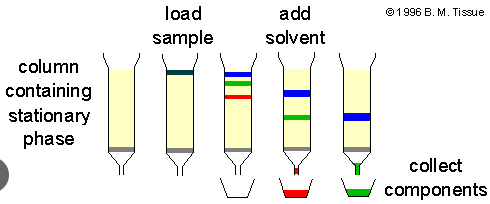
Chromatography
separation of substances based on their ability to adhere to various solids e.g paper
Physical Properties
Characteristics that can be observed w/ out altering the identity of the substance
density, color, melting point, state of matter
Chemical Properties
Characteristics of a substance that cannot be observed without altering the substance
flammability
Physical Change
Does not alter the identity of the substance
phase change
Chemical Change
Alters the identity of the substance
burning
Indications of Chemical change
Transfer of energy, change in color, production of a gas, formation of a precipitate, change in pH
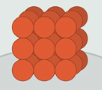
Solid
Definite shape & volume, not easily compressed, strong forces of attraction between particles

Liquid
Indefinite shape, definite volume, not easily compressed, weak forces of attraction between particles

Gas
Indefinite shape, indefinite volume, easily compressed, no attraction between particles
Sublimation
Solid to Gas
Deposition
Gas to Solid
Melting
Solid to Liquid
Freezing
Liquid to Solid
Vaporization
Liquid to Gas
Condensation
Gas to Liquid