Chemistry (only formulas)
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
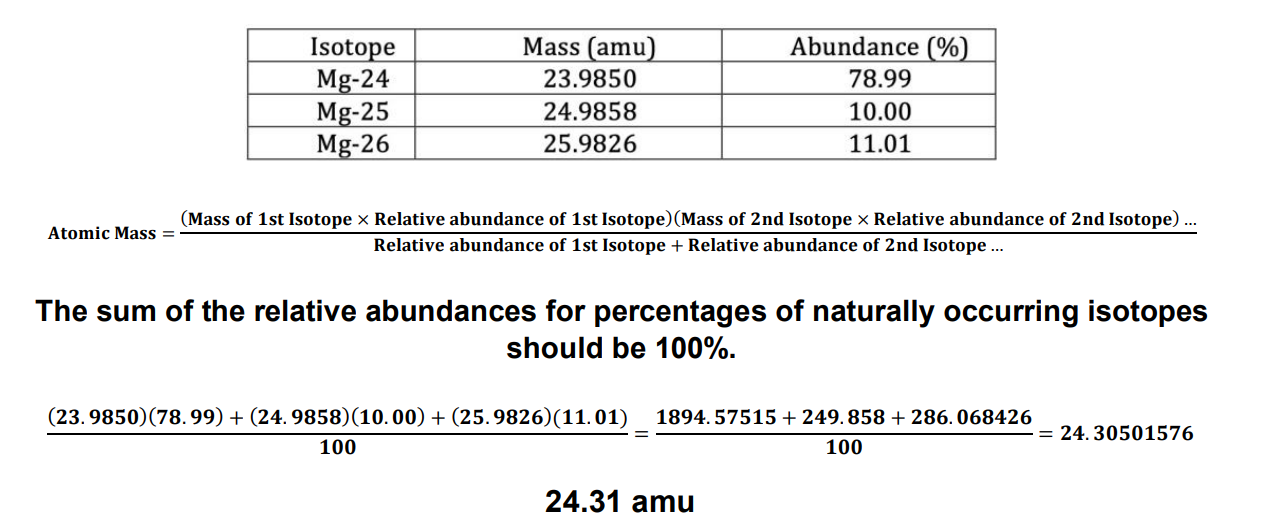
How to find atomic mass with isotope and abundances
(Mass #1)(% #1) + (Mass #2)(% #2) /100
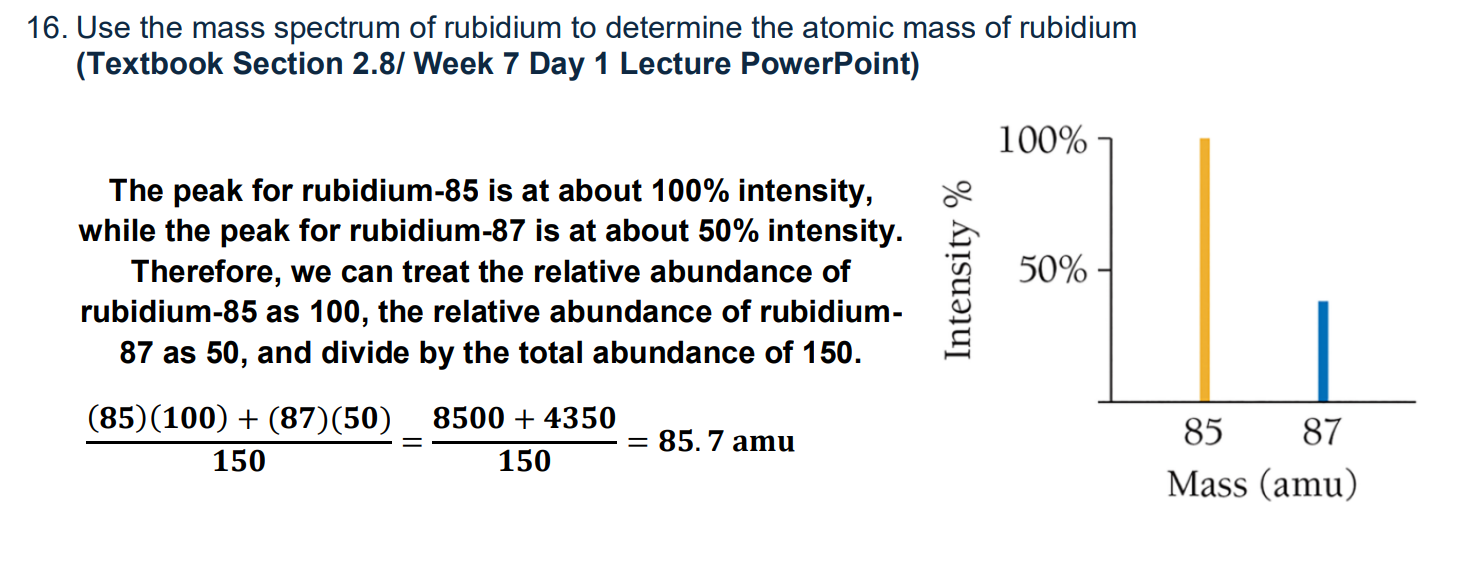
How to find atomic mass with mass spectrum image
(Mass #1)(% #1) + (Mass #2)(% #2) /sum of percentages
How to calculate how many moles are in ______ atoms?
Atoms → Moles = Divide by Avogadro's number (6.022 ×1023)
How to calculate how many moles from a given element mass
Mass → Moles = Divide by molar mass.
How to calculate how many atoms from a given element mass.
Mass → Atoms = First divide by molar mass, then multiply by Avogadro’s number.
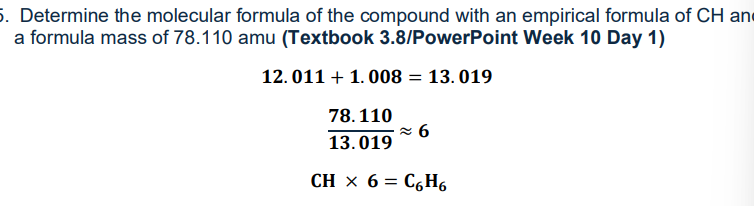
How to find the molecular formula
Formula mass/ Empirical Formula
How to calculate how many molecules are from the given mass of a compound
Given mass of compound/molar mass of compound x Avragados number (6.022 × 1023)
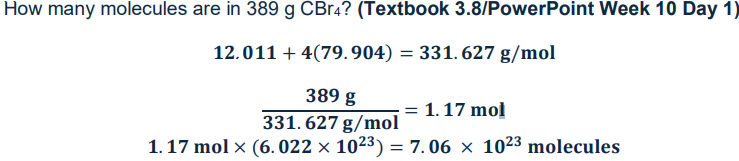
How to find percent composition of a element from a given compound
Total mass of the element we are trying to find/ Molecular mass x100
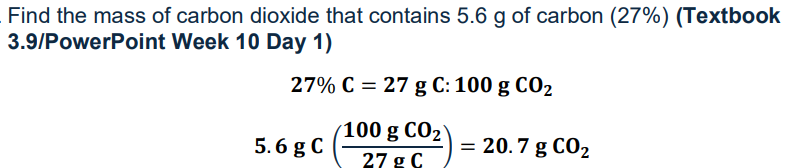
How to calculate mass of compound when given mass of element and percentage composition
mass of element/ percentage x 100