Chemistry 1.8- Thermodynamics
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
What are standard conditions?
Temperature- 298 K
Pressure- 100 kPa
Enthalpy of formation- definition + symbol
The enthalpy change when 1 mole of a compound is formed from its elements in standard states
ΔHfꝋ
Usually exothermic (-)
Enthalpy of combustion- definition + symbol
The enthalpy change when 1 mole of a substance undergoes complete combustion in oxygen with all substances in standard states
ΔHcꝋ
Always exothermic (-)
Enthalpy of neutralisation- definition + symbol
The enthalpy change when 1 mole of water is formed in an acid-alkali reaction under standard conditions
ΔHneutꝋ
Always exothermic (-)
First ionisation enthalpy- definition + symbol
The enthalpy change when each atom in 1 mole of gaseous atoms loses an electron to form 1 mole of gaseous 1+ ions
ΔHie1ꝋ
Always endothermic (+) as energy is needed to overcome the attraction between an electron and the nucleus
Second ionisation enthalpy- definition + symbol
The enthalpy change when each ion in 1 mole of gaseous 1+ ions loses an electron to form 1 mole of gaseous 2+ ions
ΔHie2ꝋ
Always endothermic (+) (more than the first ionisation) as more energy is needed to remove an electron from a positive ion
First electron affinity- definition + symbol
The enthalpy change when each atom in 1 mole of gaseous atoms gains an electron to form 1 mole of gaseous 1- ions
ΔHea1ꝋ
Always exothermic (-) as the electrons are attracted to the positive nucleus
Second electron affinity- definition + symbol
The enthalpy change when each ion in 1 mole of gaseous 1- ions gains an electron to form 1 mole of gaseous 2- ions
ΔHea2ꝋ
Always endothermic (+) (unlike the first affinity) as energy has to be put in to overcome the repulsion between the electron and the negative ion
Enthalpy of atomisation- definition + symbol
The enthalpy change when 1 mole of gaseous atoms is formed from an element in its standard state
ΔHatꝋ
Always endothermic (+)
Enthalpy of hydration- definition + symbol
The enthalpy change when 1 mole of gaseous ions is dissolved in water
ΔHhydꝋ
Always exothermic (-)
Enthalpy of solution- definition + symbol
The enthalpy change when 1 mole of an ionic solid is dissolved in water to form an infinitely dilute solution
ΔHsolꝋ
Can be exothermic (-) or endothermic (+)
Bond dissociation enthalpy- definition + symbol
The enthalpy change when 1 mole of covalent bonds is broken in the gaseous state
ΔHdisꝋ
Always endothermic (+) as energy has to be put in to break the covalent bonds
Lattice enthalpy of formation- definition + symbol
The enthalpy change when 1 mole of an ionic solid is formed from its constituent ions in the gaseous state
ΔHLEFꝋ
Always exothermic (-) as energy is released when ionic bonds are formed due to the strong electrostatic forces of attraction between the oppositely charged ions
Lattice enthalpy of dissociation- definition + symbol
The enthalpy change when 1 mole of an ionic solid is broken up into its constituent ions in the gaseous state
ΔHLEDꝋ
Always endothermic (+) as energy has to be put in to overcome the strong electrostatic forces of attraction between oppositely charged ions
Enthalpy of vapourisation- definition + symbol
The enthalpy change when 1 mole of a liquid is turned into a gas
ΔHvapꝋ
Always endothermic (+)
Enthalpy of fusion- definition + symbol
The enthalpy change when 1 mole of a solid is turned into a liquid
ΔHfusꝋ
Always endothermic (+)
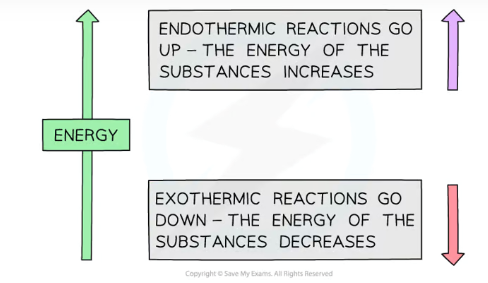
Which way do exothermic and endothermic arrows point in Born-Haber cycles?
Endothermic- up (the energy in the substances increases)
Exothermic- down (the energy in the substances decreases)
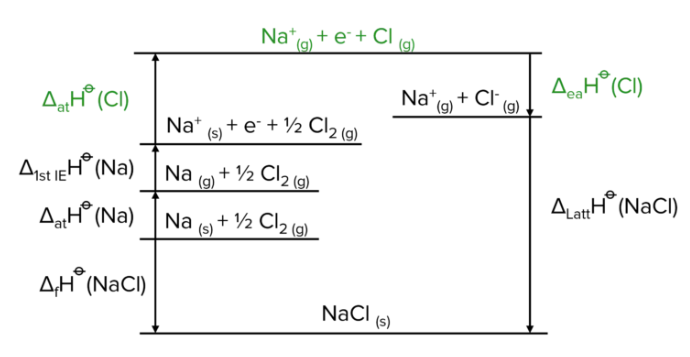
What is the general structure of a Born-Haber cycle to find the lattice enthalpy?
Starting from the bottom line (ionic solid)- NaCl(s):
The ionic solid is converted into its constituent elements in standard states (reverse of the enthalpy of formation)- Na(s) + ½Cl2 (g)
The metal in its standard state is converted into a gaseous atom (enthalpy of atomisation)- Na(g) + ½Cl2 (g)
The metal is ionised to form a 1+ ion and an electron (enthalpy of ionisation)- Na+(g) + e– + ½Cl2 (g) (this may happen multiple times to form 2+ or 3+ metal ions)
The non-metal in its standard state is converted into a gaseous atom (enthalpy of atomisation)- Na+(g) + e– + Cl (g) (this is the top line of the diagram)
The non-metal gains the electron to form a 1- ion (electron affinity)- Na+(g) + e– + ½Cl2 (g) (this may happen in two steps to form 2- ions but the second step has to be a down arrow as 2nd electron affinity is endothermic)
The gaseous ions form back into the ionic solid (lattice enthalpy of formation)- NaCl(s) (sometimes the arrow goes up instead as the lattice enthalpy of dissociation is used)
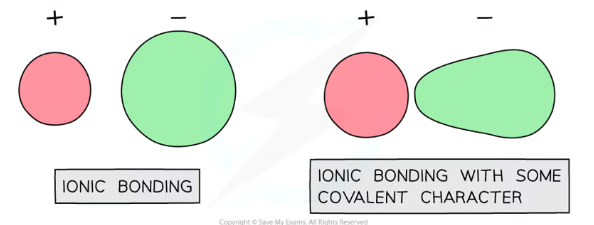
What increases the polarising ability of metal ions in a lattice? How does this affect the bond character?
Metal ions are better at polarising if they have:
Greater charges
Smaller ionic radii
This makes the metal ions better at attracting electron density towards themselves, distorting the electron clouds in an ionic lattice so that they aren’t perfectly spherical- this means the compound shows some covalent character (uneven sharing of electrons)
What increases the polarisability of non-metal ions in a lattice? How does this affect the bond character?
Non-metal ions are more easily polarised if they have:
Greater charges
Larger ionic radii
This makes it easier for the metal ions to attract electron density towards themselves, distorting the electron clouds in an ionic lattice so that they aren’t perfectly spherical- this means the compound shows some covalent character (uneven sharing of electrons)
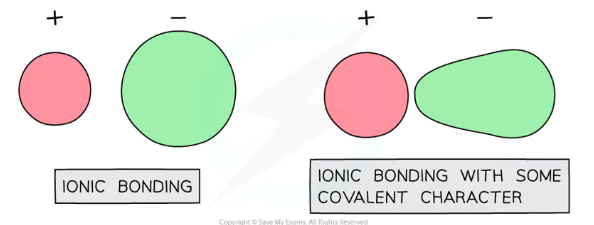
Why do ionic compounds show some covalent character? How does this affect lattice enthalpy?
Certain metal ions are better at polarising, and certain non-metal ions are more easily polarisable
This makes it easier for metal ions to attract electron density towards themselves, distorting the electron clouds in an ionic lattice so that they aren’t perfectly spherical- this means the compound shows some covalent character (uneven sharing of electrons)
Ionic compounds with more covalent character will show greater lattice enthalpy values than expected compared to theoretical calculations, so they will have a greater percentage difference between the experimental value and the theoretical value
What properties do ionic compounds with more covalent character show?
Lower solubility in water
Lower melting points
Less electrical conductivity
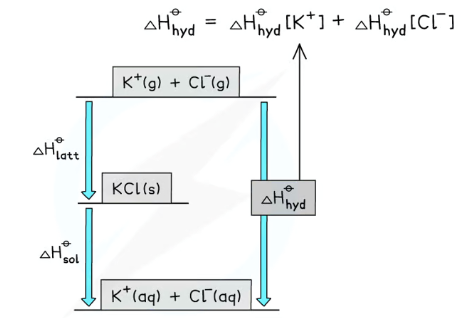
What is the structure of a solution cycle?
There are two routes to go from the top (gaseous ions) to the bottom (dissolved ions):
Direct route: enthalpy of hydration- the gaseous ions are dissolved in water (the enthalpies for each ion have to be added together)
Indirect route: lattice enthalpy of formation + enthalpy of solution- the gaseous ions are turned into an ionic solid, then the ionic solid is dissolved in water
These cycles can be used to find unknown values- check whether lattice enthalpy of formation (-) or dissociation (+) is provided and whether the enthalpy of solution is positive or negative
What is entropy?
The entropy (S) of a system is the number of different arrangements of the particles and their energy
This means it’s a measure of how disordered or variable a system is
Systems with higher entropies will be more energetically favourable as the energy is better spread out
What changes in a reaction cause entropy to increase?
Changes in state from solid to liquid to gas- increases the freedom of the particles, so they have more ways to arrange
Increases in the number of moles- having more moles of products than reactants means the number of arrangements has increased
What are the units for enthalpy and entropy?
Enthalpy = kJ mol-1
Entropy = J mol-1 K-1
What is the formula for the entropy change in a system?
ΔSsystem = ΣSproducts - ΣSreactants
What is the Gibbs free energy equation?
ΔG = ΔHreaction - TΔSsystem
ΔG = Gibbs free energy (kJ mol-1)
ΔH = enthalpy change (kJ mol-1)
T = temperature (K)
ΔS = entropy change (J K-1 mol-1 - must be converted into kJ by dividing by 1000)
How can the Gibbs free energy equation determine whether a reaction is feasible or not?
When ΔG is negative or zero, the reaction is feasible and likely to occur
When ΔG is positive, the reaction is not feasible and unlikely to occur
When will a reaction be feasible or unfeasible, judging by the signs of ΔH and ΔS?
Since temperature is measured in Kelvin, it can never be negative, so the sign of T doesn’t have to be considered (though its magnitude may affect whether ΔG is positive or negative)
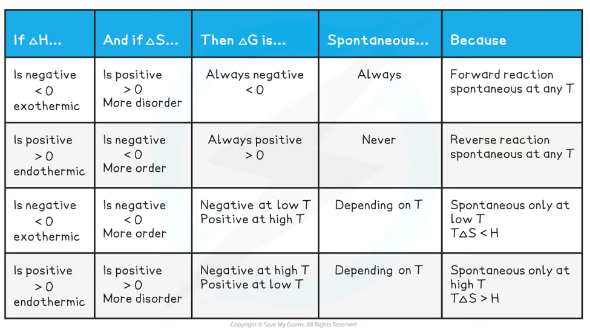
How can we use the Gibbs free energy equation to find the temperature at which a reaction becomes feasible?
Rearrange ΔG = ΔH - TΔS to T = (ΔH - ΔG) / ΔS
For a reaction to be feasible ΔG must be less than or equal to zero, so ΔG can be removed so that the equation becomes T = ΔH / ΔS
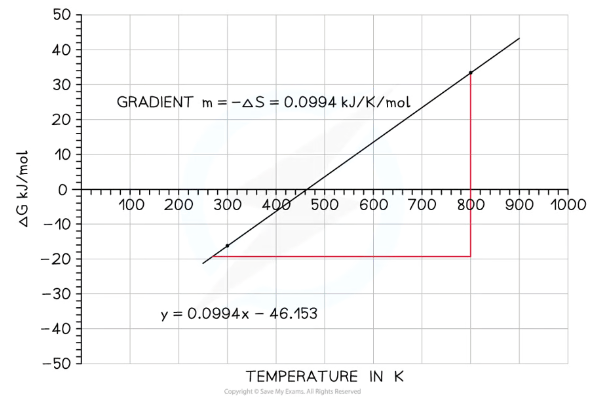
What can the graph of the Gibbs free equation illustrate?
This is the Gibbs free energy graph for an exothermic reaction
As temperature increases, ΔG becomes less negative and so the reaction becomes less feasible
The point where the line intercepts the x-axis is the point of feasibility- the temperature at which this reaction stops becoming feasible