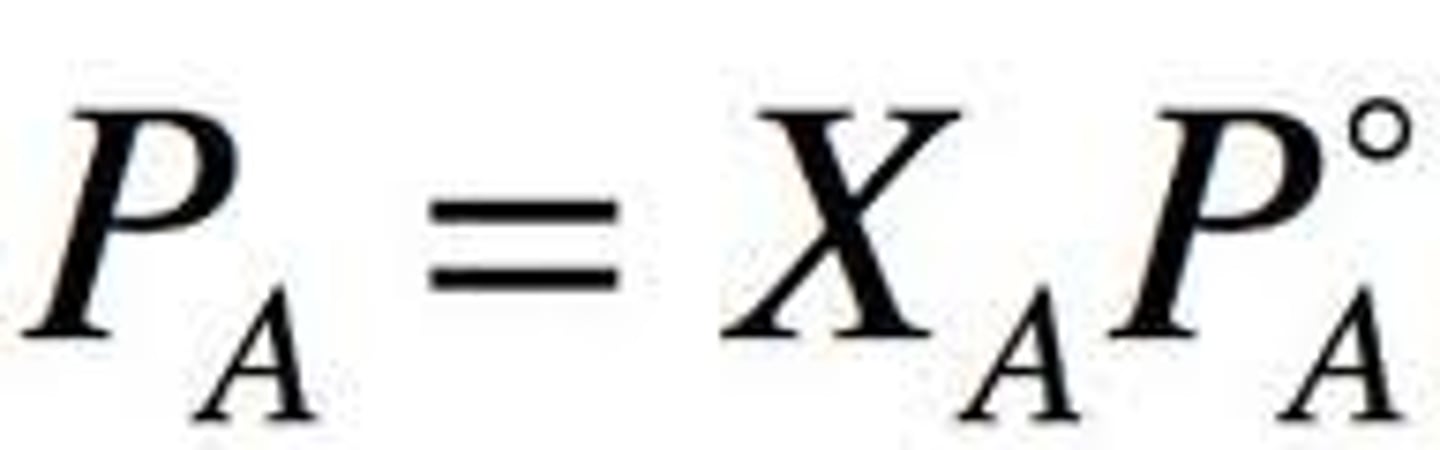PHA615 LAB: Simple and Fractional Distillation
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
It is a process of separating components of a liquid mixture
Distillation
Two processes involved in separation technique
• Vaporization of liquids
• Condensation of the resulting vapor
the force exerted by the liquid molecules per unit area of the gas at the liquid-gas interphase
Vapor Pressure
temperature at which the vapor pressure of the liquid is equal to the atmospheric pressure
Boiling Temperature
it cools the vapor causing it to liquify and directs the condensate into the receiving flask.
Condenser
promote even healing of the liquid (it is added before heat is applied to the distilling flask
Boiling Stones
filled with loosely packed inert substance (glass beads) that increases the distance traveled by the vapor and the surface area available for condensation.
Fractionating Column
Types of Distillation
1. Simple Distillation
2. Fractional Distillation
3. Steam Distillation
4. Vacuum Distillation
How distillation happens?
1. Heat is slowly applied to the distillation flask.
2. As vapor rises from the liquid, it raises the temperature of the apparatus.
3. The vapor condenses and drips into the receiving flask (15-20 drops/min).
4. The temperature stabilizes at the boiling point and most of the liquid distills over into the receiving flask.
5. When the temperature drops, distillation is halted.
The graph produced is a boiling point as a function of composition for mixtures of ethanol and butanol
Distillation of Mixtures
Dalton's Law of Partial Pressures
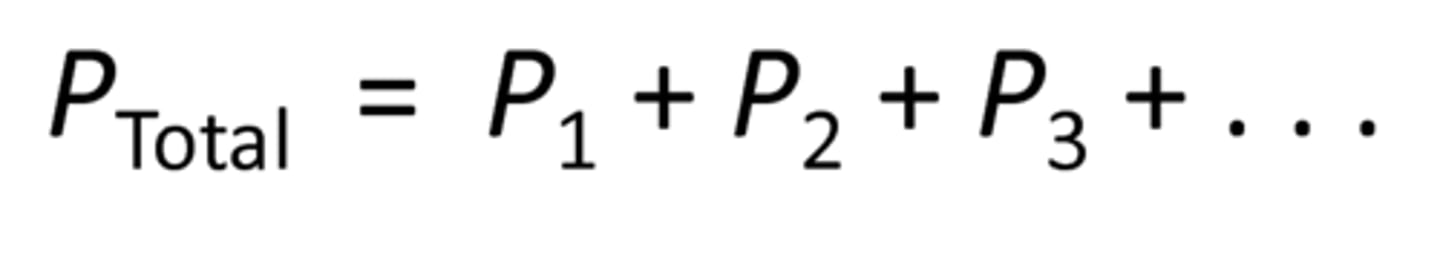
Raoult's Law
Pa=XaPa0