Electronic Structure of Atoms
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
electronic structure
number of electrons in the atom, their distribution around the nucleus, and their energies
electromagnetic radiation (radiant energy)
form of energy that has wave characteristics
wavelength (λ)
distance between two peaks or troughs
m, meters
frequency (ν)
number of complete wavelengths that pass through a certain point
must be in Hz, s^-1
speed of light (c)
= 3.00 x 10⁸ m/s
quantity at which all types of electromagnetic energy moves
equation for electromagnetic radiation
c = λν
(c = 3.00 x 10⁸ m/s, ν = frequency in s⁻¹, λ = wavelength in m)
what did Maxwell Planck suggest about energy
energy could be emitted or absorbed in increments/levels
quantum
refers to the smallest quantity of energy that can be emitted or absorbed as electromagnetic radiation
equation for quantum energy
E = hν
(E = energy of single quantum, h = 6.626 x 10⁻³⁴ J-s, ν = frequency)
what does h stand for
Planck's constant, 6.626 x 10⁻³⁴ J-s
be able to recognize, don't need to memorize
how does hv relate to matter
matter can emit or absorb energy in whole number multiples of hv
photoelectric effect
metals emit electrons when light is shined on them
what is required for photoelectric effect to occur
minimum frequency of light is required and is different for each metal
photon
energy packet of light
equations for photon energy
E = hv
E = hc/λ
what do photons do
photons transfer energy to the electrons; if photons have enough energy, the electrons can escape from the metal
summary of light equations
electromagnetic radiation: c = λν
quantum energy: E = hν
photon energy: E = hv, E = hc/λ
spectrum
made when radiation from polychromatic light source is split into its component wavelengths with a prism
continuous spectrum
contains almost all wavelengths/colors (ex. rainbows)
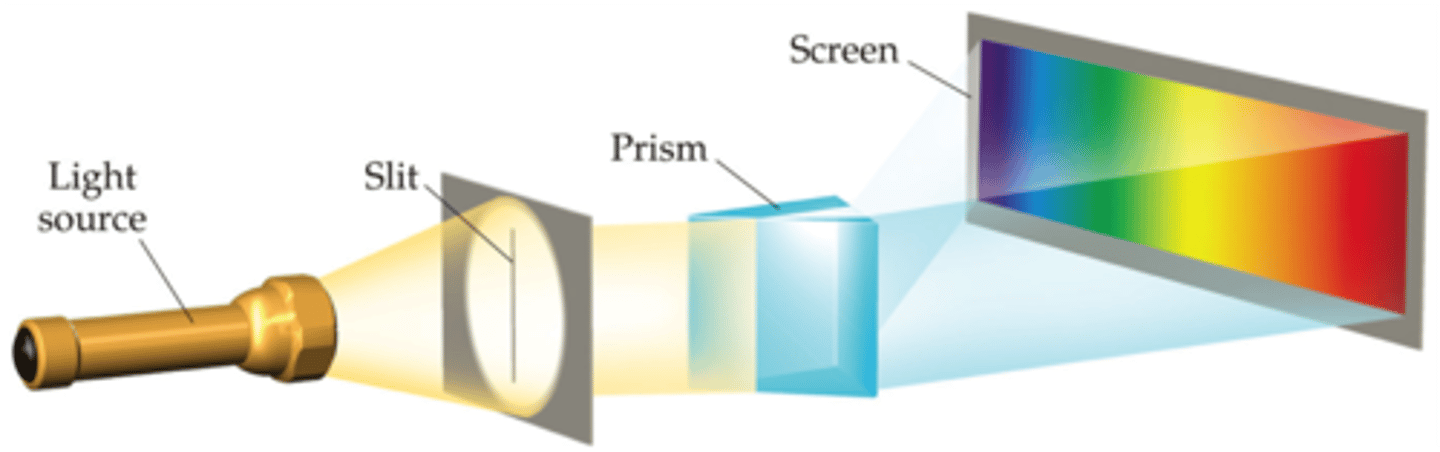
line spectra
contain only a few specific wavelengths/colors (ex. when voltage is applied to a gas under low pressure, neon lights)
Postulates for H atom (Bohr Model of the Atom)
1. Only orbits of certain radii, corresponding to specific energies, are permitted for the electron is a H atom.
2. An electron in a permitted orbit is in an "allowed" energy state. In this state, it does not radiate energy and does not spiral into nucleus.
3. Energy is emitted or absorbed by the electron only as the electron changes from one allowed energy state to another. This energy is emitted or absorbed as a photon that has energy E = hν.
equation for energies corresponding to the allowed orbits for the electron in H atom
E = (-2.18x10⁻¹⁸ J)(1/n²) = (-hcRH)(1/n²)
n = principal quantum number (whole number)
principle quantum number
each allowed orbit corresponds to different value of n
radius increases as n increases
ground state
closest state to the nucleus (n = 1)
lowest energy and therefore the most stable (most energy)
excited states
everything that isn't ground state (n = 2+)
higher energy, less stable states
reference or zero energy state
when electron is separated from the nucleus (n = infinity)
what do electrons do when they change energy states
electrons will absorb or emit radiant energy
equation for change in energy
ΔE = Ef - Ei
ΔE = (-2.18x10-18 J)(1/nf2 - 1/ni2)
+ΔE = e- absorb energy to move to a higher state/n.
-ΔE = e- emit energy to move to a lower state/n.
how is energy absorbed or emitted as
photons
+ΔE (nf > ni);
ΔE = hν = Ephoton
photon absorbed
-ΔE (nf < ni);
- ΔE = hν = Ephoton
photon emitted
what tells you whether the photon is absorbed or emitted since h and v are positive
the sign
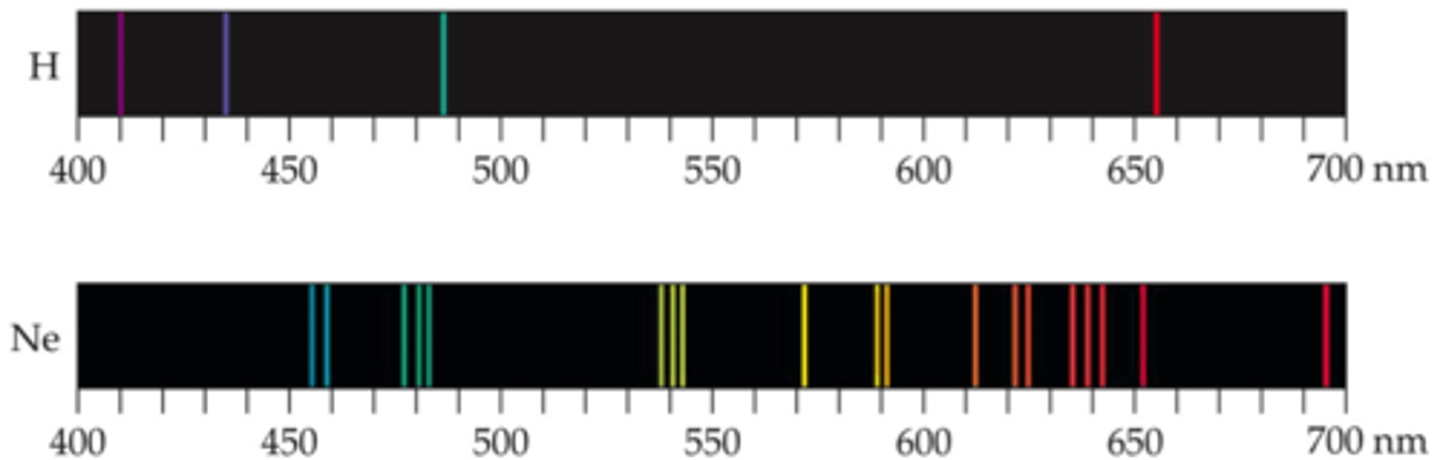
the lines in a line spectra can be attributed to
quantized jumps of electrons between energy levels
limitations of Bohr model
only fully explains a hydrogen atom
assumes that electron wouldn't fall into nucleus
electron has wave-like properties; doesn't just "circle" the nucleus
significance of Bohr's model
electrons only exist in discrete energy levels, which are described by quantum numbers
energy is involved in the transition of an electron from one energy state to another
what did Louis de Broglie suggest that electrons could behave like
a wave, more specifically matter waves
matter waves equation
λ = h / (mv)
m = mass in kg
v = velocity in m/s
mv = momentum
Heisenberg's uncertainty principle
states that it is impossible for us to know simultaneously both the exact momentum and exact position of particles with a small mass (i.e. electrons).
ncertainty of position: Δx ≥ h/(4πmΔv)
x = position
quantum mechanics
an approach to electronic structure that incorporates both wave-like and particle-like behaviors of the electron
Schrodinger proposed a
wave equation and wave functions (ψ) that treat electrons as standing waves that haves nodes or points where the magnitude of the wave is 0.
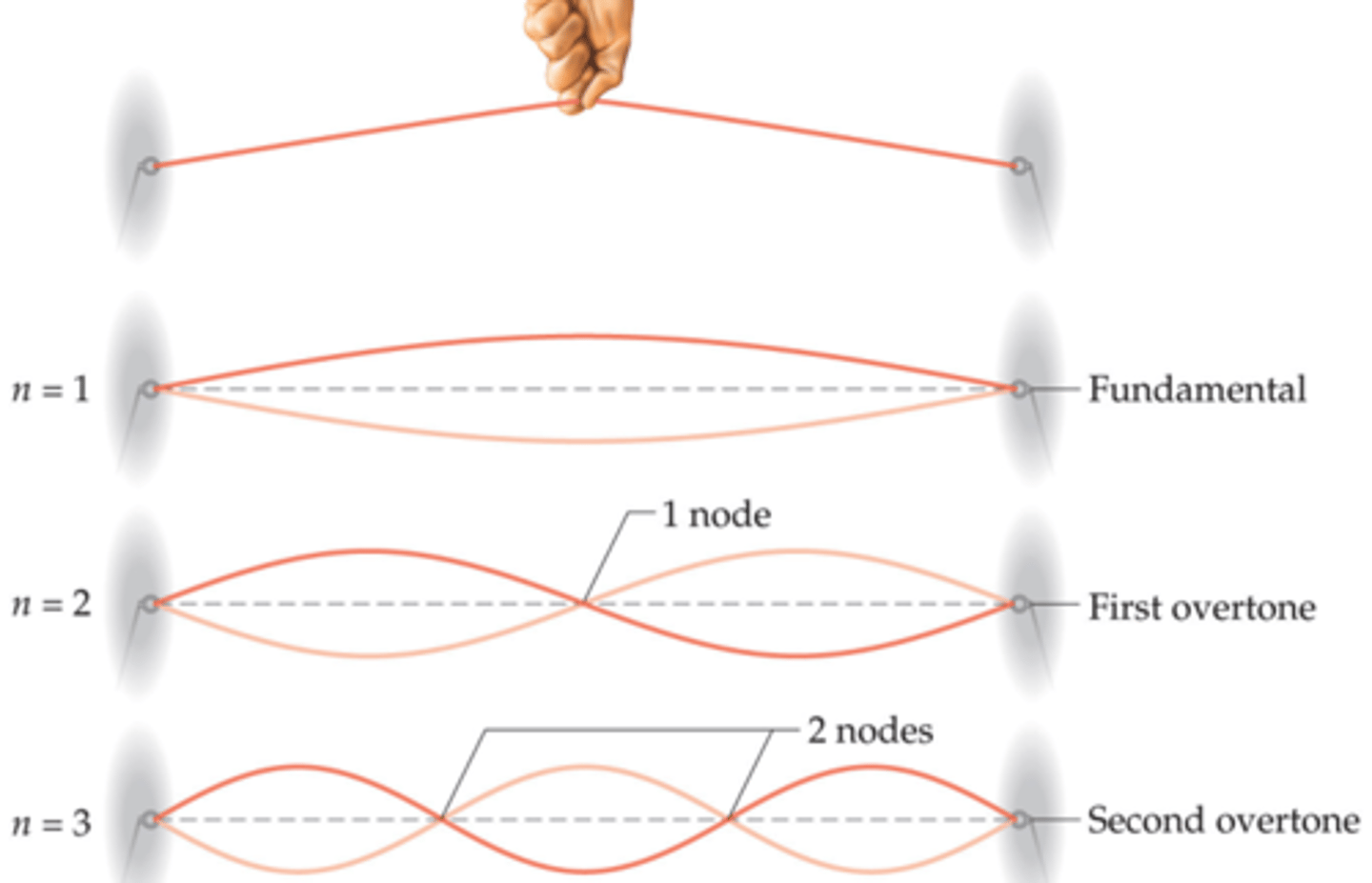
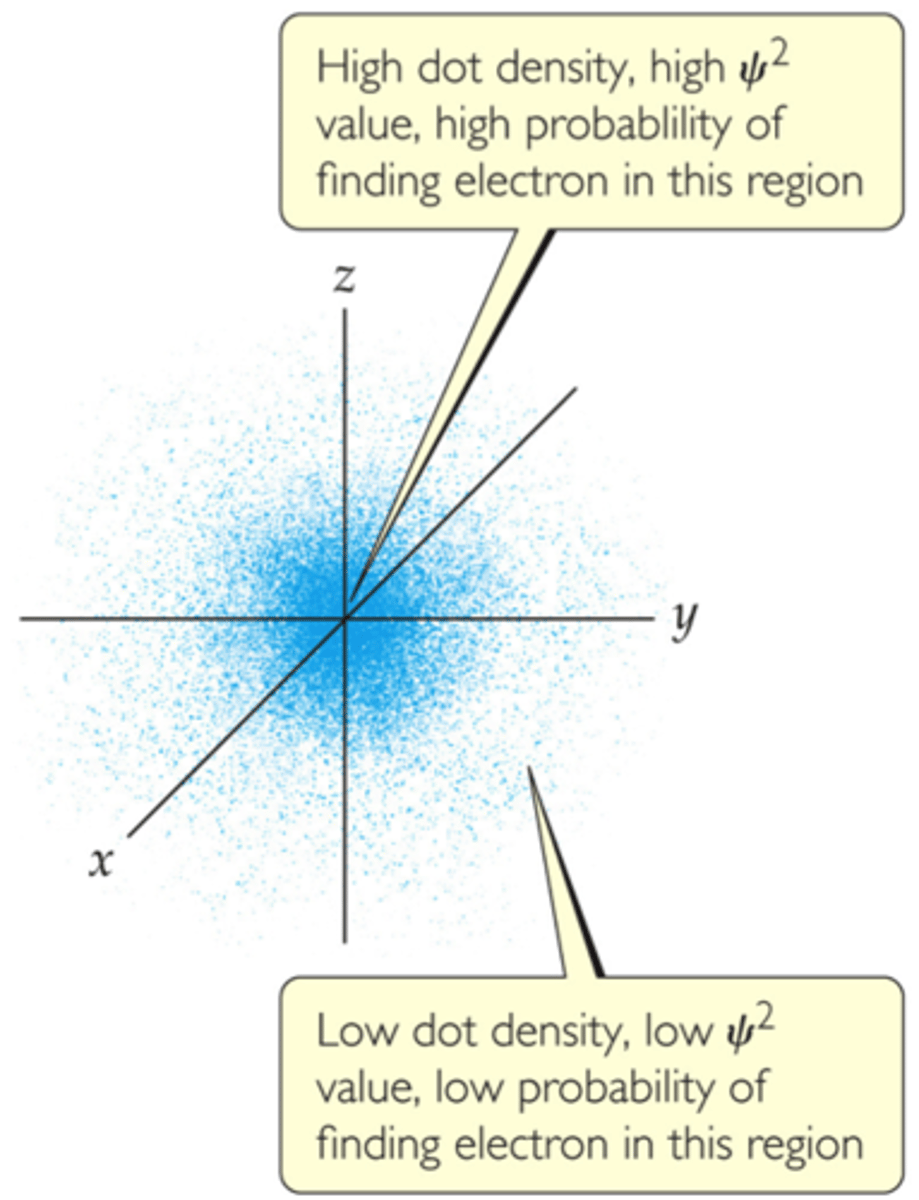
probability or electron density (ψ2)
represents the probability that an electron will be found at some location
orbitals
regions within an atom where electrons are most likely to be found
quantum numbers are
used to describe the characteristic shape and energy of each orbital
n, l, ml, s
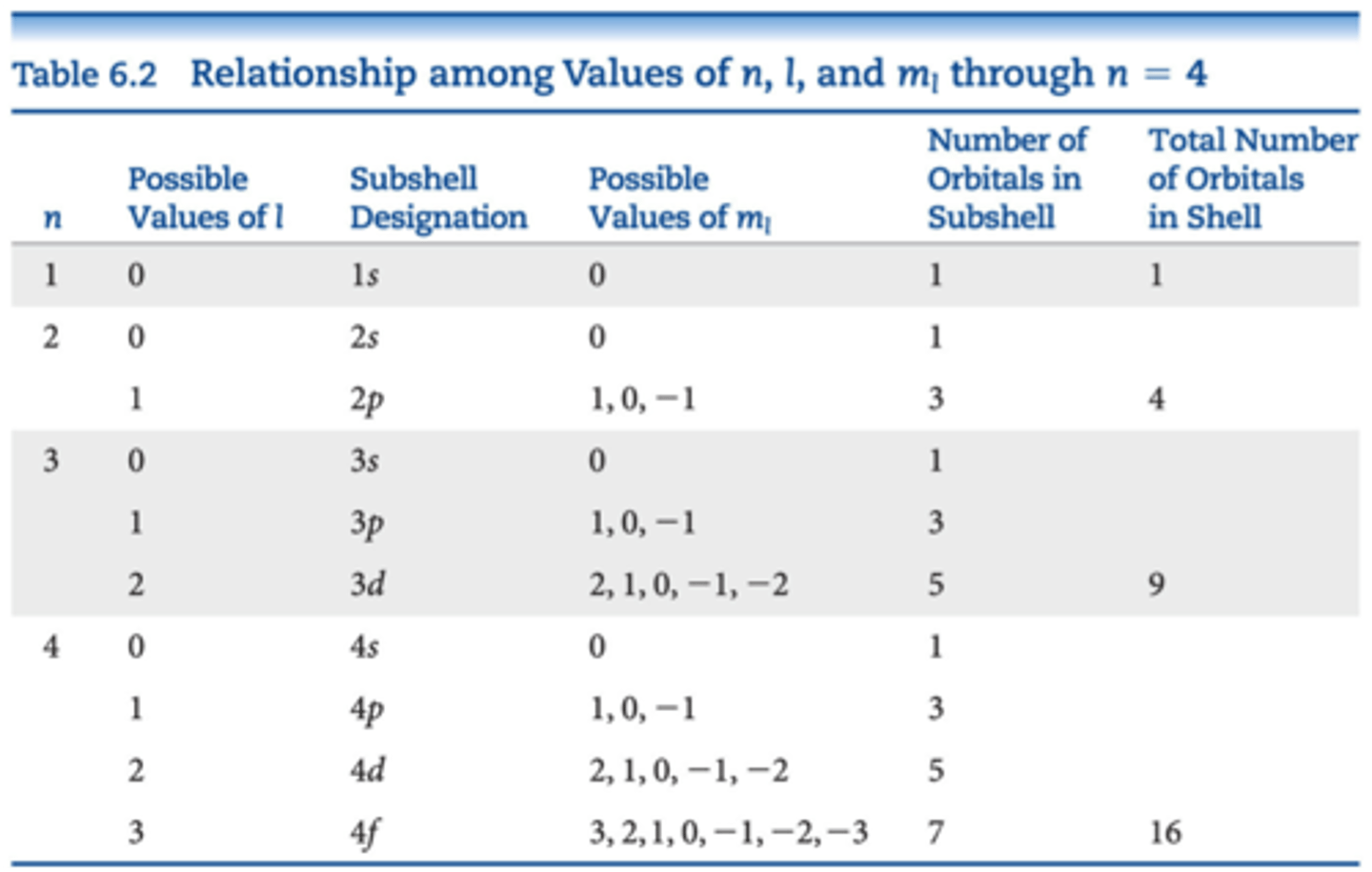
n
principal quantum number
same principles from Boh's model apply
En = -2.18 x 10^-18 J (1/n^2)
l
angular momentum quantum number
values range from 0 to (n-1) for each n
defines the shape of the orbital
usually designated by letters
ml
magnetic quantum number
values are whole numbers between -l and l including 0.
describes the orientation of the orbital
ms
spin number
describes the spin
1/2 or -1/2
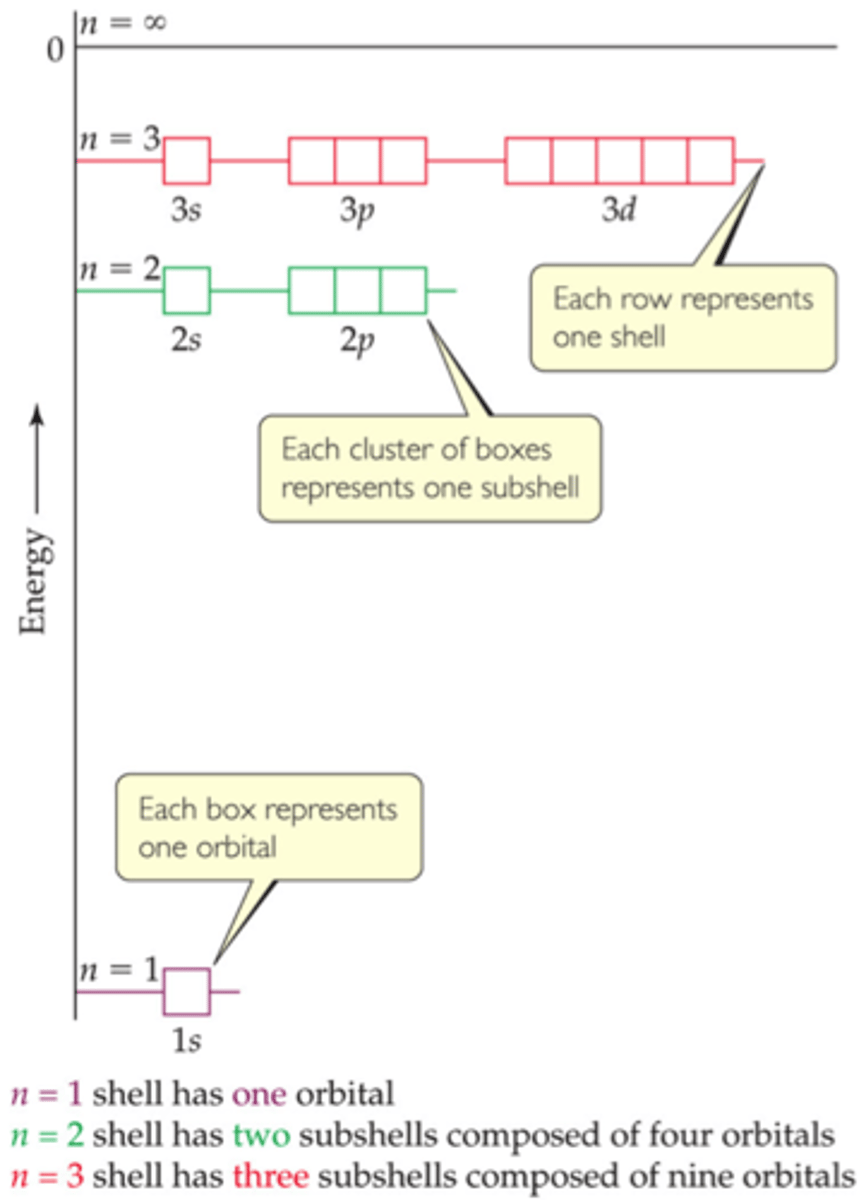
electron shells
collections of orbitals with the same n
subshells
have same n and l values
designated by a number and a letter
a shell only contains n number of subshells
each subshell consists of a specific number of orbitals
the total number of orbitals in a shell is n^2
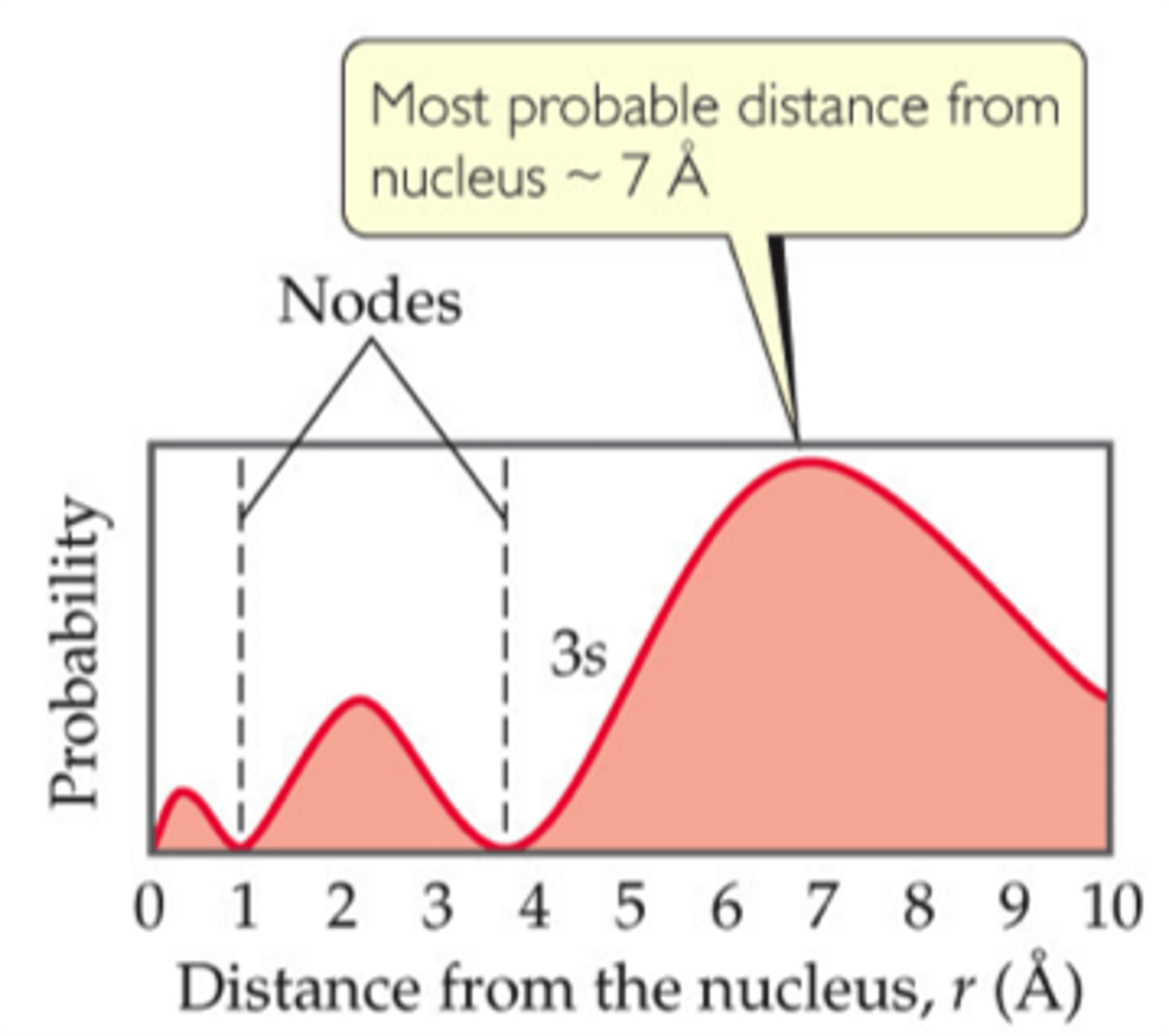
radial probability function
shows how far away an electron is likely to be from the nucleus
The number of peaks is equal to n. The outermost peak is the largest.
The number of nodes is equal to n-1.
As n increases, there is a greater probability that the electron is farther from the nucleus so the size of the orbital increases.
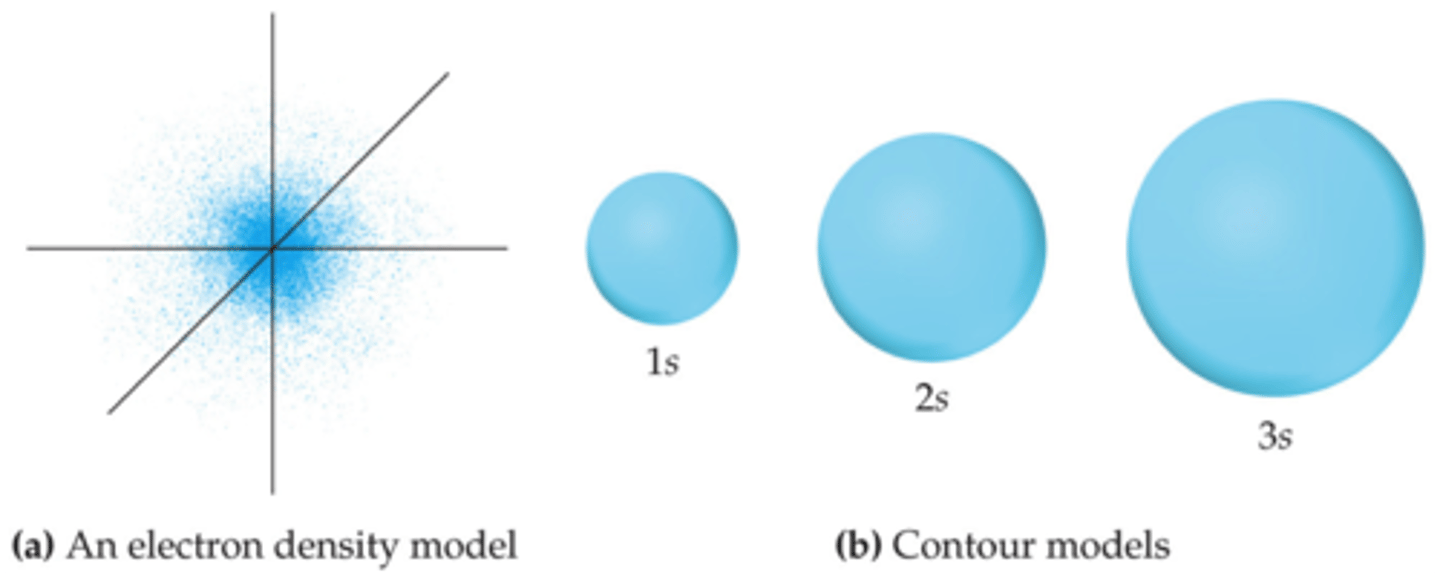
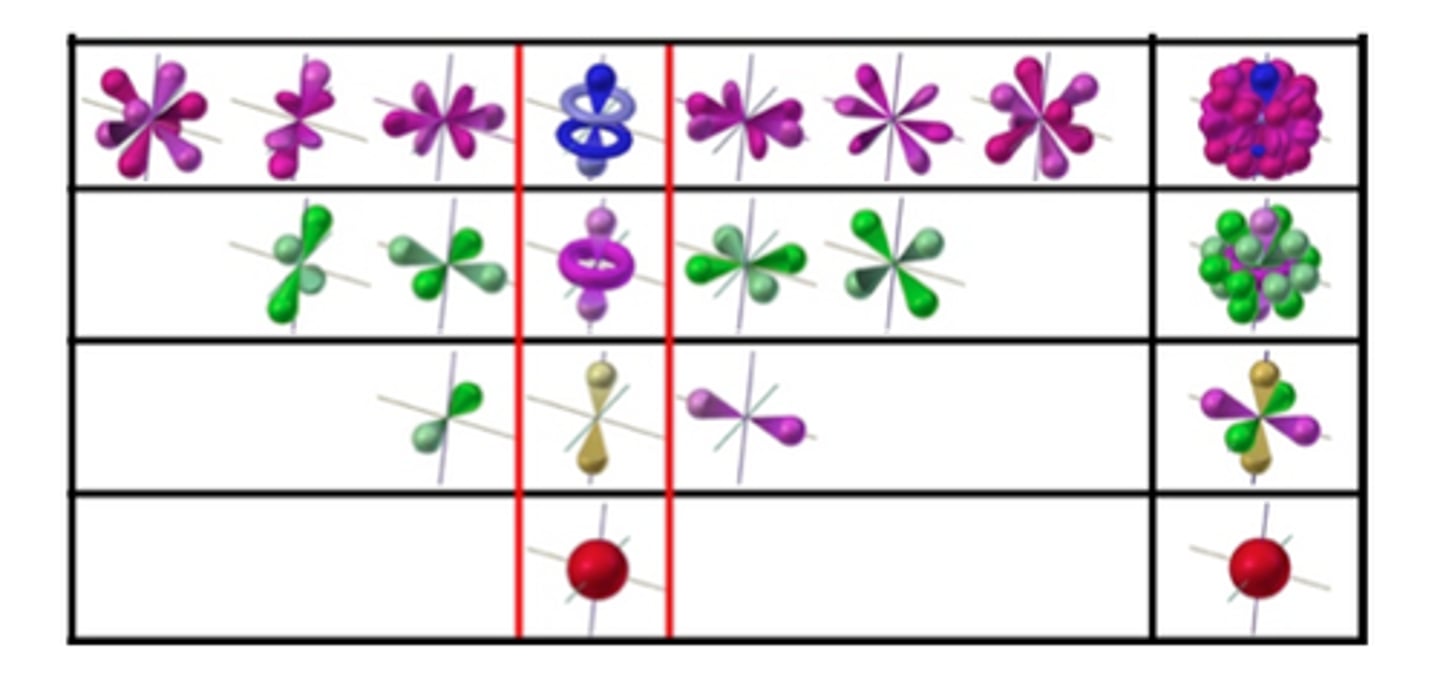
s orbital
spherically symmetric
each shell has one s orbital
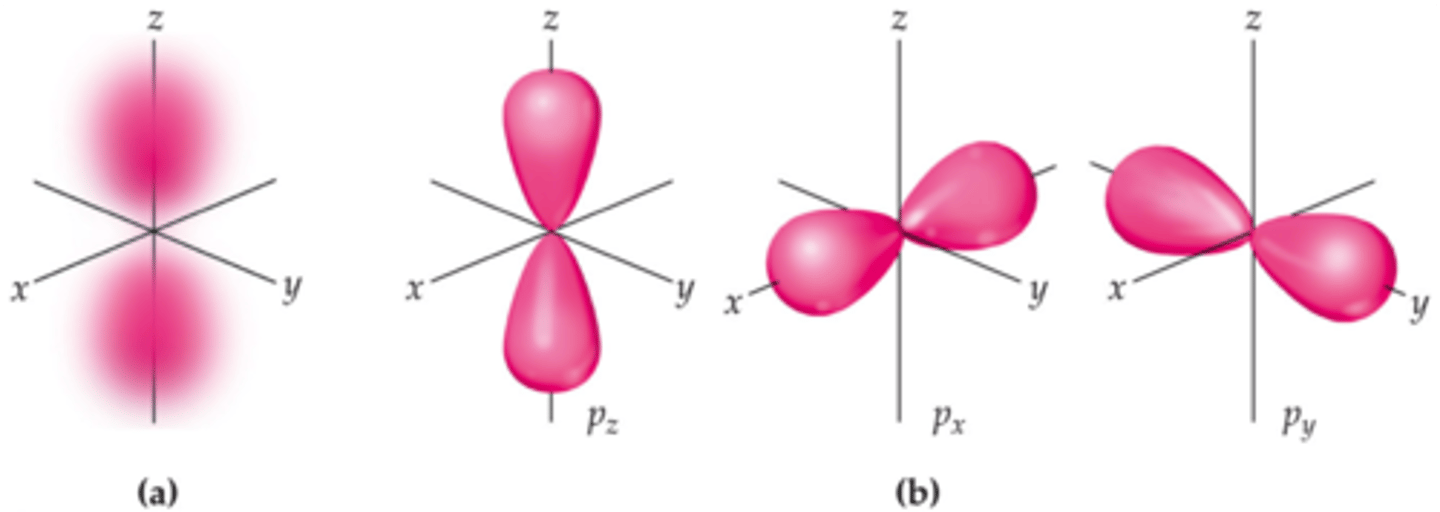
p orbital
dumbbell-shaped (two dense regions or lobes on either side of the nucleus with a node in the middle)
each shell with p orbitals has three p orbitals of the same size but different orientation
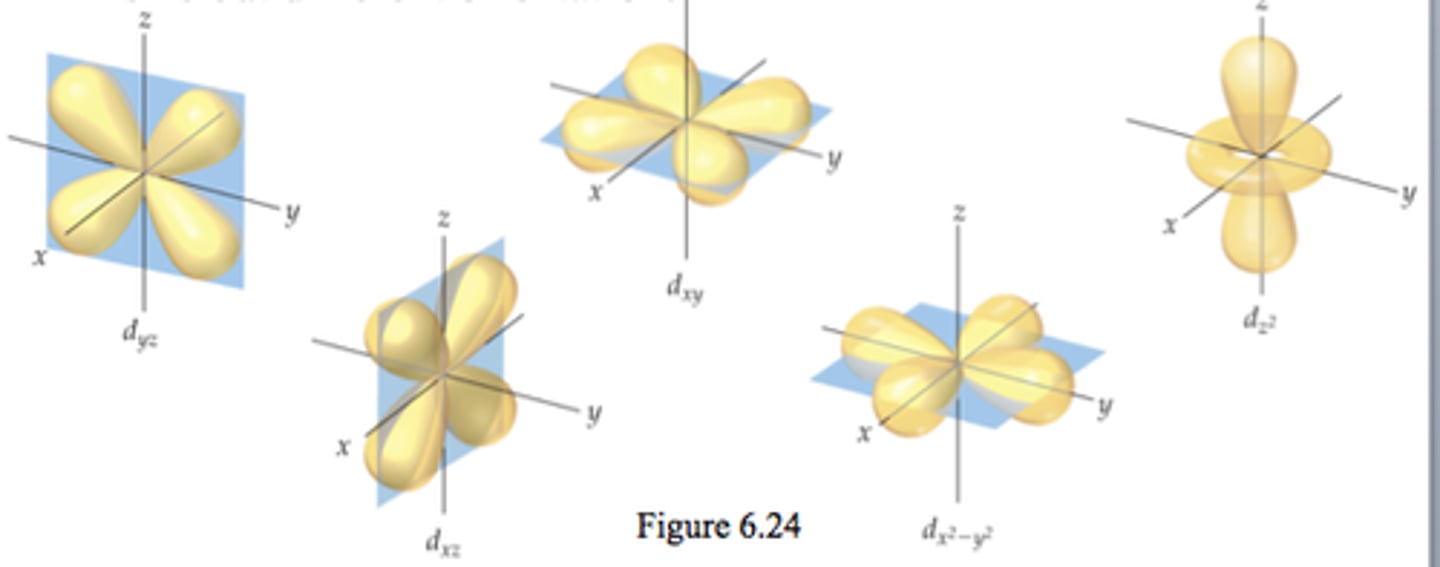
d orbitals
vary in shape
each shell with d orbitals has five d orbitals of the same size but different orientation
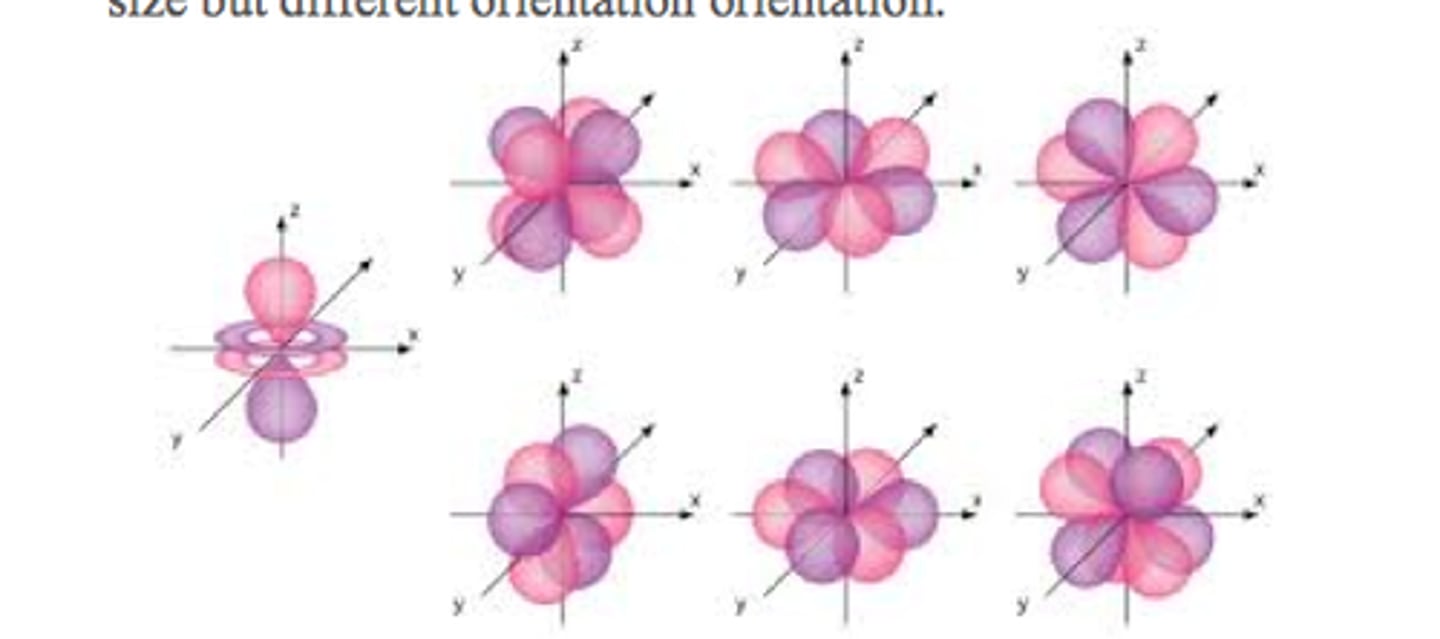
f orbitals
vary in shape
each shell with f orbitals has seven f orbitals of the same size but different orientation
representations of orbitals
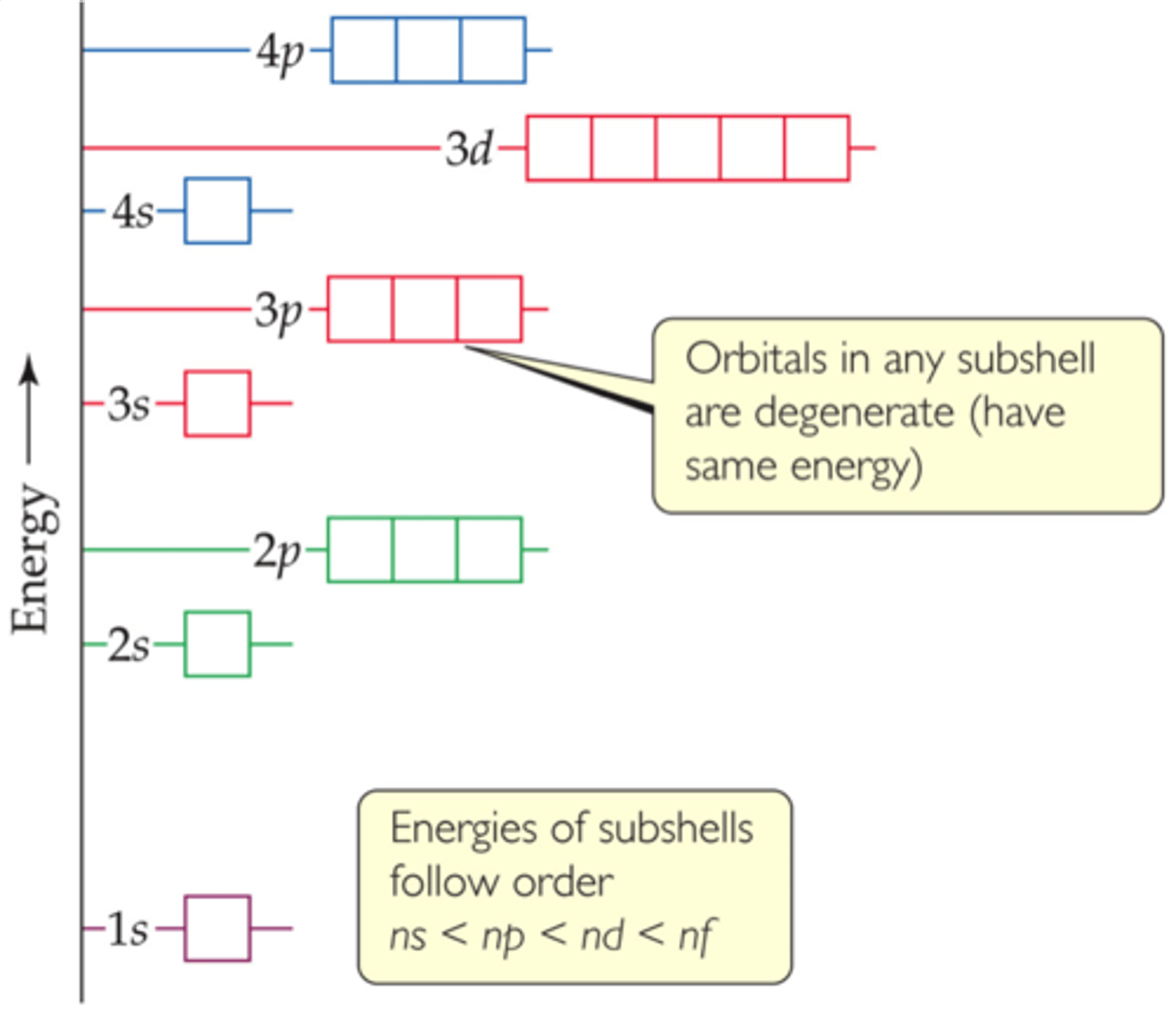
many-electron atoms
The electronic structure of many-electron atoms can be described by using the orbitals described for H, but their energies are different.
For a given n, the energy of an orbital increases with increasing l.
All orbitals in a given subshell have same energy and are said to be degenerate.
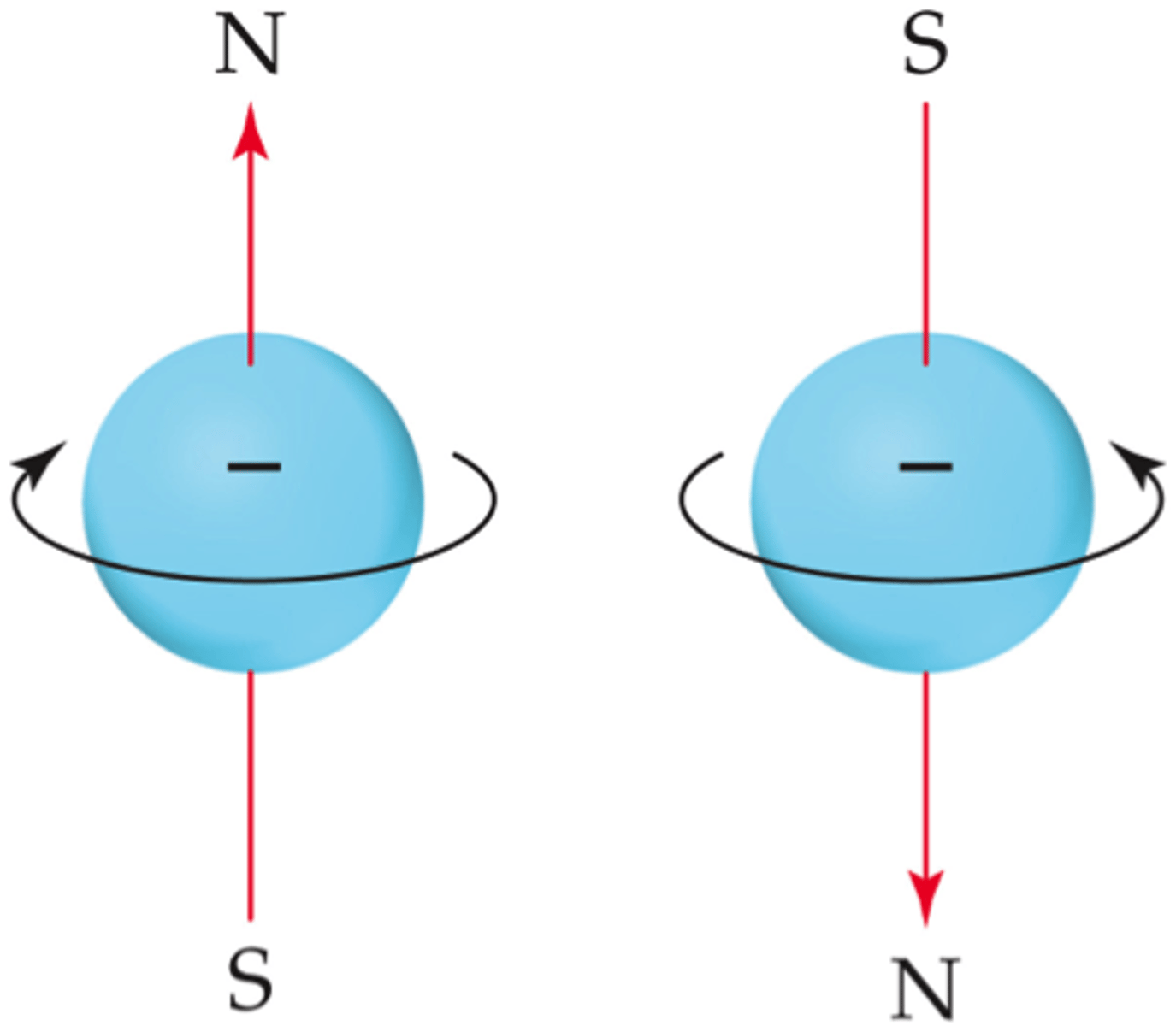
electron spin
intrinsic property of electrons to spin on its own axis; quantized value
ms = spin magnetic quantum number
values are +1/2 and -1/2
Pauli exclusion principle
states that no two electrons in an atom can have the same set of four quantum numbers
for a given orbital, n, l, and ml are fixed; only ms can change
actual important part: therefore an orbital can hold a maximum of two electrons with must have opposite spins
electron configuration
distribution of electrons among that various orbitals
orbitals filled in order of increasing energy with no more than 2 electrons per orbital
ground state for many-electron atoms
orbital diagrams
used to represent electrons configurations
paired electrons have opposite spins and occupy the same orbital
unpaired electron in a lone electron in an orbital
Hund's rule
first they fill up orbitals before they pair up
core electrons
inner shell electrons and are equivalent to the electron configuration of the preceding noble gas
outer shell electrons
those listed after noble gas
valence electrons
outer shell electrons involved in chemical bonding