#23: The Secretory Pathway & Protein Quality Control
1/85
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
86 Terms
What 2 types of proteins go through the secretory pathway?
secreted proteins, integral membrane proteins
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
hormones
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
LDL
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
serum blood albumin
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
blood clotting proteins
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
antibodies
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
snot (mucus)
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
connective tissue proteins
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
digestive enzymes
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
spider silk
secreted
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
ion channels
integral membrane
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
nutrient transporters
integral membrane
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
hormone receptors
integral membrane
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
neurotransmitter receptors
integral membrane
Is this an example of a SECRETED protein or an INTEGRAL MEMBRANE protein?
cell adhesion molecules
integral membrane
___% of the genes in your genome encode membrane proteins
30
Secreted and integral membrane proteins are _____________ ___________ into and through _____ and ______ compartments, then transported to ______ destinations.
sequentially transported, ER, Golgi, final
Palade’s Pulse-Chase Experiment
What was his question?
What type of cells did he study to answer this question?
What route do proteins take through a cell?, guinea pig pancreas exocrine cells (they secrete digestive enzymes)
Palade’s Pulse-Chase Experiment
Cellular process?
Pulse?
Chase?
protein secretion via the secretory pathway, radioactive leucine (³H-Leucine), normal leucine (¹H-Leucine)
Palade’s Pulse-Chase Experiment
Data Collection
Took __________ ______________ (a photograph taken using an electron microscope) of the cells at various time points where the ____________ __________ appear as black dots
Measured the % of ______________ at each location after the ______ round and 3 rounds of ______.
electron micrographs, radioactive proteins, radioactivity, pulse, chase
Palade’s Pulse-Chase Experiment
Where was the radioactivity located in the electron micrograph taken immediately after the pulse?
Why does this make sense?
on the rough ER, this is where proteins are being newly synthesized with the radioactive leucine amino acids
Palade’s Pulse-Chase Experiment
Where was the radioactivity located in the electron micrograph taken 37 minutes after the pulse?
in vesicles outside of the ER
Palade’s Pulse-Chase Experiment
% Radioactivity Results:
Pulse (3 mins) = 86.3% of radioactivity located on the ________ _____
Chase (+7 mins) = 43.0% of radioactivity located in the ___________ _________ of the ______
Chase (+37 mins) = 48.5% of radioactivity located in the ______________ _________ of the ______
Chase (+117 mins) = 58.6% of radioactivity located in __________ ________
rough ER, periphery vesicles, Golgi, condensing vacuoles, Golgi, secretory vesicles
Palade’s Pulse-Chase Experiment
What was the conclusion?
proteins go from the rough ER to the Golgi to secretory vesicles
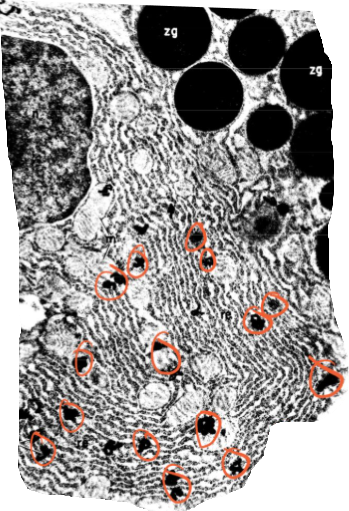
Palade’s Pulse-Chase Experiment
Is this the electron micrograph taken immediately after the pulse or 37 minutes after during the chase?
immediately after the pulse
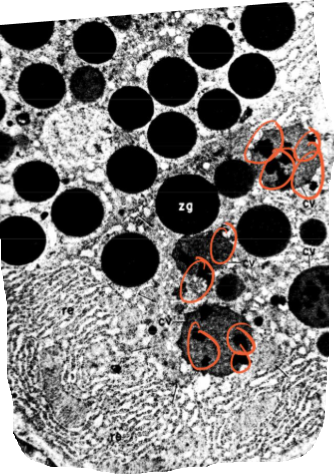
Palade’s Pulse-Chase Experiment
Is this the electron micrograph taken immediately after the pulse or 37 minutes after during the chase?
37 minutes after
The Big Questions in Membrane Trafficking
How do compartments keep their ________ with all the flow of ___________ in and out?
How to maintain the _____ of compartments?
How to _____ with the ______ membrane and not with the ______ one? (___________)
How is trafficking directed between ______________?
How to overcome the ________ ________ of fusion?
identity, membrane, size, fuse, right, wrong, specificity, compartments, energy barrier
What does it mean for the components of the secretory pathway to be “topologically equivalent”?
whatever is on the inside of the vesicle becomes part of the extracellular environment
What is the central organelle of the secretory pathway?
What is its nickname?
Golgi, the soul of the cell
Rothman’s Golgi Trafficking Complementation Assay
What was his question?
How does protein trafficking through the Golgi work?
Rothman’s Golgi Trafficking Complementation Assay
The Problem: Within the Golgi, trafficking occurs _________ different ______ of ___________, specifically from the ____→ _______→ _____Golgi. We want to be able to ________ the Golgi in a lab setting to be able to study the pathway. It turns out that we can isolate the _______ ______, but we can’t actually isolate the _____________ _________.
between, stacks, membranes, cis, medial, trans, isolate, whole Golgi, individual stacks
a test to identify and measure the amount of a specific molecule in a sample
assay
Rothman’s Golgi Trafficking Complementation Assay
Rothman isolated 2 different Golgi preparations from 2 different cell lines: mutant and wild type. The mutant cell line expressed the ______ _________ _______ in the _____ Golgi but lacked the ________ needed to _____________ the protein in the ______ Golgi (³H-GlcNAc glycosyltransferase). The wild type cell line did express this _________ in the ______ Golgi but he did not _______ the _____ Golgi with the ______ __________ ________. This meant that on their own, neither cell line would produce ______________ ___________.
Rothman mixed the 2 preparations until he found a situation that worked. Mixing together the ______ Golgi from the mutant cell line and the ______ Golgi from the wild type cell line _______________ the ______________ _______ upon a successful transport → aka ______________ ___________ were produced!
viral protein VSV-G, cis, enzyme, glycosylate, medial, enzyme, medial, infect, cis, viral protein VSV-G, glycosylated proteins, cis, medial, complemented, glycosylation defect, glycosylated proteins
Rothman’s Golgi Trafficking Complementation Assay
He was able to figure out what is needed for proteins to be able to move between the Golgi ______ to get _____________, ultimately realizing that both ____ (i.e. ________) and the ___________ (i.e. other ____________ in the _____) are needed for Golgi trafficking.
stacks, glycosylated, ATP, energy, cytoplasm, molecules, cell
Rothman’s Golgi Trafficking Complementation Assay
Process of Membrane Trafficking: General Scheme
cargo __________ → vesicle __________ → vesicle __________ → vesicle _________ → vesicle ________
selection, binding, transport, docking, fusion
Rothman’s Golgi Trafficking Complementation Assay
What is one of the factors that he discovered that is required for protein movement through the Golgi and assists with vesicle fusion?
SNARE proteins
Rothman’s Golgi Trafficking Complementation Assay
Specificity: different _________ and different ________ ___________ have different ________ proteins that only work in specific combinations
vesicles, target membranes, SNARE
What is the job of the ER? (7 examples)
protein folding, disulfide bond formation, proteolytic cleavage, formation of multimeric complexes, passing things to the Golgi, lipid synthesis, regulation of cholesterol synthesis
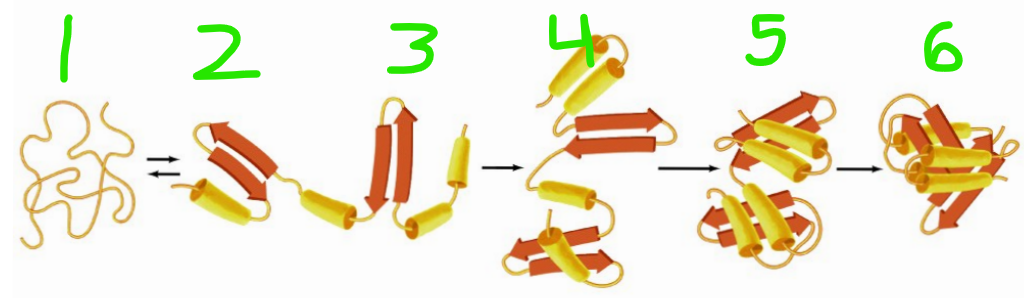
Which of these is in the lowest energy state?
6
Protein Folding
Recall the _____________ _______, where proteins fold to achieve the _______ _______ ______ with the hydrophobic parts on the _______ and the hydrophilic parts on the _______
hydrophobic effect, lowest energy state, inside, outside
Protein Folding
Why is protein folding particularly difficult in the ER? (2)
What helps with protein folding in the ER?
denser concentration of proteins, limited space, chaperones
Protein Folding
One of the most abundant chaperones in the ER
BiP
Protein Folding
As the unfolded protein is being threaded through the __________, _____ grabs onto it and helps it bind and unbind the _____________ parts to help them find their ____-_______ states
translocon, BiP, hydrophobic, low-energy
Protein Folding
Which chaperone also helps to pull the unfolded proteins into the translocon to begin with?
BiP
Protein Folding
The chaperone ____ helps form and rearrange _________ _______, trying different combinations until the protein reaches its ________ ________ state
PDI, disulfide bonds, lowest energy
Protein Folding
when proteins clump together to form insoluble structures
aggregation
Protein Folding
Protein aggregation can result from WHAT?
protein misfolding
Protein Folding
Which type of proteins are particularly susceptible to aggregation?
neurons
Protein Folding
Name 3 examples of protein misfolding diseases.
What do they all have in common?
Alzheimer’s, Huntington’s, Parkinson’s, protein aggregation
Protein Folding
What is the trade-off between? (2)
Which disease is an example of the consequences of this trade-off?
accuracy, time, cystic fibrosis
Cystic Fibrosis
The cystic fibrosis gene encodes a _________ _________. The inability to pass _________ _____ through can lead to a _______ __________________ in our _________. It turns out that the most common mutation in this gene is a ____ ________ on the protein far out from the actual ________. People who express the gene with this mutation are able to create a perfectly ________ _________ ________ protein. The problem is that the protein is _____ to _____. This makes our cells think that the protein could ______________, causing our cells to _________ the protein. This is a good mechanism for preventing ________ _____________, but in this case it causes cystic fibrosis.
chloride channel, chloride ions, mucus accumulation, airways, Phe deletion, channel, normal folded channel, slow, fold, aggregate, degrade, protein aggregation
Protein Folding
What is one clue that might hint to a cell that a protein could aggregate?
slow folding
N-Linked Glycosylation as Quality Control
The __________ of the oligosaccharide on proteins in the ___ ______ into the 5-residue pentasaccharide core starts by removing the ___ _________ _________. It turns out that trimming the _____ _________ is a _____________ step that is used to determine whether a protein is ________ or not.
trimming, ER lumen, 3 terminal glucoses, final glucose, reversible, folded
N-Linked Glycosylation as Quality Control
The chaperone __________ helps with the ________ of the protein and removes the _____ _________ residue from the oligosaccharide. The protein without _________ is now assessed to see if it is ___________ ________. If it is, it _______ the ___. If it is not, it __-_______ the cycle. ________ ____________ recognizes unfolded proteins and will __-________ the ________ if the protein is not completely ________. The ___________ protein now goes back through the ________-removal process with __________ to try again.
Calnexin, folding, last glucose, glucose, properly folded, leaves, ER, re-enters, Glucosyl Transferase, re-attach, glucose, folded, misfolded, glucose, Calnexin
ER Stress & the Unfolded Protein Response (UPR)
To reduce stress in the ER, what could we do to the amount of misfolded proteins: turn it UP or turn it DOWN?
down
ER Stress & the Unfolded Protein Response (UPR)
To reduce stress in the ER, what could we do to the total translation: turn it UP or turn it DOWN?
What could this do?
down, reduce the load of misfolded proteins
ER Stress & the Unfolded Protein Response (UPR)
To reduce stress in the ER, what could we do to the synthesis of chaperones: turn it UP or turn it DOWN?
What could this do?
up, help with protein folding
Protein Folding
Name 3 examples of chaperones
BiP, PDI, Calnexin
ER Stress & the Unfolded Protein Response (UPR)
To reduce stress in the ER, what could we do to the transcription of mRNA: turn it UP or turn it DOWN?
What could this do?
What could we do to make sure the rest of the cell is unaffected?
down, reduce total protein load, only target mRNAs on the ER
ER Stress & the Unfolded Protein Response (UPR)
To reduce stress in the ER, what could we do to the ER volume: turn it UP or turn it DOWN?
What could this do?
What specific process would we turn up to do this?
up, make more room for protein folding, lipid synthesis
ER Stress & the Unfolded Protein Response (UPR)
To reduce stress in the ER, what could we do to overall cell growth: START or STOP?
stop (slow it down)
ER Stress & the Unfolded Protein Response (UPR)
What does the UPR need to be able to do? (2)
sense misfolded and unfolded proteins, turn that into a cellular response
ER Stress & the Unfolded Protein Response (UPR)
What are the 3 branches of signaling the UPR?
IRE1, PERK, ATF6
ER Stress & the Unfolded Protein Response (UPR)
Both ______ and ______ branches upregulate the transcription of XBP1 mRNA but the ______ branch is required to make the XBP1 mRNA useful by translating into a _____________ XBP1 ______________ __________.
PERK, ATF6, IRE1, functional, transcription factor
ER Stress & the Unfolded Protein Response (UPR)
_____ and _____ are both ________ that sense mis/unfolded proteins and go through _____________, _________________, and downstream signaling very much like the _________ ______.
IRE1, PERK, kinases, dimerization, autophosphorylation, insulin RTK
ER Stress & the Unfolded Protein Response (UPR)
______ is a ______________ ________ that in the absence of stress is held in the ____ but in the presence of stress is passed to the ______. When it gets the _____ ______, it travels to the ______ where it is ________ by the same __________ that cleave _______ in __________ synthesis regulation. This cleavage turns it into an _______ ____________ _______ which can now turn on things that help the ____ deal with this stress of protein ___________ like ____________ and ______ ___________.
ATF6, transcription factor, ER, Golgi, stress signal, Golgi, cleaved, proteases, SREBP, cholesterol, active transcription factor, ER, misfolding, chaperones, lipid synthesis
ER Stress & the Unfolded Protein Response (UPR)
PERK Mechanism:
The presence of _________ proteins acts as a signal for PERK to _________.
_____________ triggers ___________________ of the ________ domains.
PERK phosphorylates _____ (a ____________ __________ _______) which _________ shuts down _____________.
While almost every other protein synthesis __________, phosphorylated _____ upregulates the translation of the protein _____, which is a ____________ ________ that turns on things like ___________ to assist with protein ________.
unfolded, dimerize, dimerization, autophosphorylation, kinase, eIF2, translation initiation factor, globally, translation, decreases, eIF2, ATF4, transcription factor, chaperones, folding
ER Stress & the Unfolded Protein Response (UPR)
Name the 4 mechanisms that are apart of the UPR
IRE1, PERK, ATF6, ERAD (ER-Associated Degradation)
ER Stress & the Unfolded Protein Response (UPR)
IRE1 Mechanism:
The presence of ________ proteins acts as a signal for IRE1 to form __________, which then _________________.
Active IRE1 _________ then ________ _____ sitting on the ___________.
Active IRE1 _________ also act as an _____________:
This _________ mechanism is completely different from the ____________. The ______ mRNA has an _______ that is not recognized by the ____________, meaning that in the absence of ______, _______ does not occur and we produce an ___________ ______ transcription factor protein. In the presence of ______, IRE1 acts as an _______________, cuts out specifically that little _______, and through ligation we form a __________ ______ transcription factor protein that now turns on other stuff to help deal with the stress like ____________, ______ proteins, and ______ __________.
unfolded, oligomers, autophosphorylate, oligomers, degrade mRNA, translocon, oligomers, endonuclease, splicing, spliceosome, XBP1, intron, spliceosome, stress, splicing, unfunctional XBP1, stress, endonuclease, intron, functional XBP1, chaperones, ERAD, lipid synthesis
ER Stress & the Unfolded Protein Response (UPR)
What is a way to degrade terminally misfolded proteins in situations where we could not prevent or fix the misfolding?
What do we have to do to the proteins targeted for termination?
Why?
ER-associated degradation (ERAD), move them out of the ER into the cytoplasm, the ER does not have proteasomes
ER Stress & the Unfolded Protein Response (UPR)
When it gets too stressful, what does the cell do?
apoptosis (aka cell suicide)
The classic experiments of George Palade and his colleagues first demonstrated the sequence of organelles that constitute the secretory pathway. It is:
A. Rough ER/nucleus/secretory vesicles/cell exterior
B. Rough ER/Golgi/secretory vesicles/cell exterior
C. Rough ER/cytosol/Golgi/cell exterior
D. Rough ER/vacuole/Golgi/cell exterior
B
Which organelle receives proteins and lipids from the endoplasmic reticulum, modifies them, and then dispatches them to other destinations in the cell?
A. An endosome
B. The Golgi apparatus
C. A peroxisome
D. The nucleus
E. A mitochondrion
B
Which ONE of the following proteins does NOT enter the secretory pathway?
A. SRP
B. signal peptidase
C. SNARE protein
D. Sec61 translocon
E. All of these proteins enter the secretory pathway
A
Which of the following is true about posttranslational modifications?
A. Carbohydrate side chains are added only after complete synthesis of the polypeptide
B. Any amino acid can be modified by phosphorylation
C. All signal sequences must be removed
D. Disulfide bonds are common in proteins to be exported
D
Proteins are properly folded with the assistance of…
A. chaperones
B. signal recognition particle (SRP)
C. Sec61 translocon
D. ribozymes
A
Which of the following are used to assist in protein folding?
I. calnexin
II. calreticulin
III. Hsp70
IV. disulfide bonds
A. III, IV
B. II, IV
C. I, II, III, IV
D. I, III
E. I, II
C
Which of these diseases does not directly involve protein aggregation?
A. Cystic fibrosis
B. Parkinson’s
C. Alzheimer’s
D. Huntington’s
A
Which disease can be caused by too much ER stress?
A. Type II diabetes
B. Parkinson’s
C. Huntington’s
D. Alzheimer’s
A
The unfolded protein response (UPR) operates at which levels of regulation?
I. transcription initiation
II. mRNA decay
III. translational initiation
IV. protein degradation
A. II, IV
B. I, III
C. I, II
D. I, II, III, IV
E. III, IV
D
You perform a pulse-chase experiment where you label a newly synthesized M6P receptor with a pulse and then follow the location of the M6P receptor at different times afterwards. Which of the following BEST represents the expected sequence?
A. Cytoplasmic ribosomes, mitochondria
B. ER, Golgi, vesicles, plasma membrane
C. ER, Golgi, vesicles, outside the cell
D. ER, Golgi, vesicles, lysosome
E. ER, Golgi, vesicles, lysosome, Golgi
D (technically A and E are also right. A is the most “general” answer and E includes how the receptors ultimately get recycled back into the Golgi after they bind to the lysosome and lose their binding affinity)
If a slice of pancreatic tissue had been incubated in ³H-leucine continuously for 40 min, where would you expect to find incorporated radioactivity in acinar cells?
A. ER
B. ER, Golgi vesicles
C. ER, Golgi vesicles, condensing vacuoles
D. Zymogen granules
E. Condensing granules
C
Which of the following is NOT a way to help relieve protein folding stress in the ER?
A. Decrease translation
B. Decrease transcription
C. Increase chaperone synthesis
D. Increase lipid synthesis
E. All of the above help relieve protein folding stress
E
Mutations in PERK cause early onset diabetes. What components of UPR would you expect to be missing in beta cells lacking functional PERK?
A. Downregulation of eIF2 alpha
B. Cleavage of ATF6
C. Splicing of XPB1 mRNA
D. A & B
E. A & C
A
Which of these is NOT a function of IRE1?
A. Directly senses unfolded proteins
B. It is cleaved to produce a transcription factor that goes to the nucleus and upregulates chaperones
C. Promotes degradation of mRNA that is being translated at ribosome docked on Sec61
D. Splices the XBP1 mRNA
B
Which of these might be a possible treatment of cystic fibrosis caused by the most common genetic variant in CFTR, the cystic fibrosis chloride channel?
A. A drug that blocks the kinase activity of PERK
B. A mutation in the residues of IRE1 that are autophosphorylated
C. A drug that blocks ERAD
D. A mutation in ATF6 that prevents its cleavage by proteases
C