Strong and Weak Acids
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What are strong acids
Acids that ionise completely in water
How can we tell when an acid has fully ionised
With one arrow
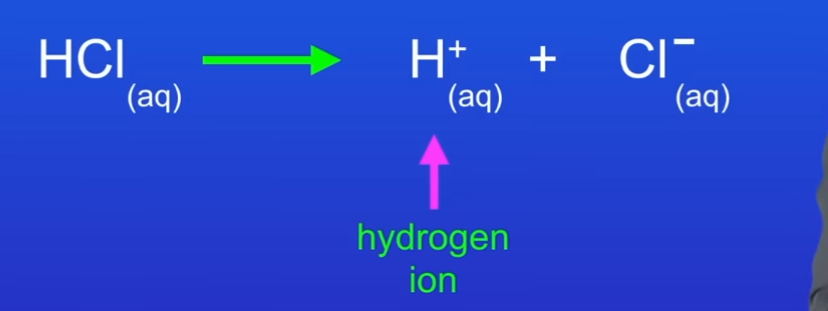
What are the three examples of strong acids
Hydrochloric acid
Sulfuric acid
Nitric acid
What are weak acids
Acids that do not ionise fully in solutions only partially
How do we know a weak acid in a equation
When arrows go both ways
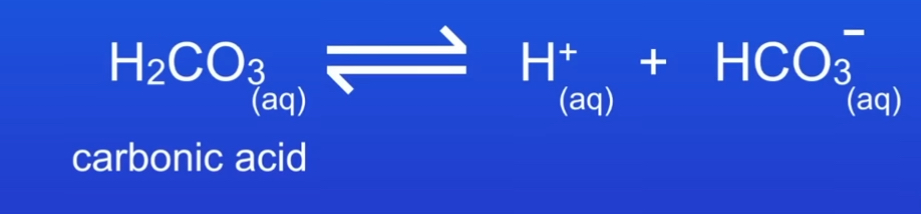
What are the three examples of weak acids
Carbonic
Ethanoic
Citric
What is the ph of strong acids compared to weak acids
Strong acids have a lower ph
What does the ph scale measure
The concentration of H+ ions in the solution
What happens when ph scale decreases by 1 unit
This means the concentration of H+ ions increases by 10 times
What is the general rule and give an example
Factor H+ ion concentration changes by = 10-x.
X is the difference in pH. So if pH falls from 7 to 4 then difference is -3 and the factor the H+ ion concentration has increased by is 10-(-3)=103
What does the concentration of a acid tell us
The amount of acid molecules in a given volume of solution
What will be a dilute acids concentration
It will have fewer molecules in a given volume compared to a concentrated acid if the strength is the same
As the acid concentration increases what happens to ph
It will decrease regardless of whether it’s a strong or weak acid