Exam Review: Chapter 6 - Energy, Enzymes and Biological Reactions
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
Entropy
the measure of molecular disorder, or randomness.
the more randomly arranged a collection of matter is, the greater the value of this value.
Free Energy
refers to the measure of a system's instability; the tendency to change to a more stable state
What is chemical energy?
Potential energy available for release in a chemical reaction
How do biologists refer to the potential energy in a chemical reaction?
Chemical energy
Electrical Energy
Energy caused by the movement of electrons.
Thermal Energy
energy contained within a system that is responsible for its temperature
Radiant Energy
the transfer of energy through electromagnetic waves; transmits energy by emitting waves that can travel through space, heating objects they come in contact with
Surroundings
everything outside of the system
1st Law of thermodynamics
law of conservation of energy, energy can be transformed from one form to another, or transferred from one place to another, but it cannot be created or destroyed
What happens when a system moves toward equilibrium
its free energy becomes progressively lower and reaches its lowest point when the system achieves equilibrium (∆G=0)
How does an exergonic (catabolic) reaction proceed?
proceeds with a net release of free energy. ∆G is negative for an exergonic reaction.
What determines the function of a protein?
the 3-d structure of a protein's conformation; each enzyme has a specific protein structure that catalyzes a specific reaction
Enzyme Denaturation
a process that occurs when an enzyme loses its shape and isn't able to bind to a substrate and catalyze a reaction
Enzyme inhibitors
nonsubstrate molecules that bind to an enzyme and decrease its activity
reversible enzyme inhibition
inactivates an enzyme through non-covalent, reversible interactions
uncompetitive inhibitor
binds to the enzyme-substrate complex, preventing release of products
Bioenergetics
focuses on how cells transform energy, often by producing, storing or consuming adenosine triphosphate (ATP)
Kinetic Energy
energy of an object in motion; energy associated with the relative motion of objects
Example of kinetic energy
a falling rock, electricity, and light
-moving objects can perform work by impairing motion to other matter
potential energy
stored energy
Examples of potential energy
a rock at the top of a hill, chemical energy, gravitational energy, and stored mechanical energy
System
the object under study
Isolated System
is unable to exchange either energy or matter with it surroundings outside
ex: in the case of approximated liquid in a thermos bottle, the liquid is unable to exchange energy or matter with the outside of the thermos
Closed System
this type of system can exchange energy (as heat or work) but not matter, with its surroundings
ex: earth is considered to be the best example of this as it on transfers energy but not matter around its atmosphere
Open Systems
energy and matter can be transferred between the system and its surroundings
ex: all livings things are considered this type of system; they absorb energy, like light or chemical energy organically, and they release heat and metabolic waste products like carbon dioxide to its surroundings
2nd Law of Thermodynamics
entropy, the total disorder (entropy) of a system and its surroundings always increases (although the total energy in the universe does not change)
The portion of a system's energy that is available to do work is called
Free Energy (G)
What does Gibbs free energy determine?
if a process can occur spontaneously or not
Define Spontaneous Reaction.
A chemical or physical reaction that will occur without an input of energy.
What determines if a reaction tends to occur spontaneously?
If the entropy of the products is greater than the entropy of the reactants.
nonspontaneous reaction
a process that, on its own, leads to a decrease in entropy ; only happens if energy is supplied
For a reaction to be spontaneous
ΔG must be negative; negative enthalpy (∆H), dominates in making a reaction spontaneous
Gibbs free energy change
∆G=∆H-T∆S
Are organisms open or closed systems?
Open systems; materials flow in and out, keeping metabolic pathways from ever reaching equilibrium, and the cell continues to do work throughout its life
how does an endergonic (anabolic) reaction act?
it absorbs free energy from its surroundings; ∆G is positive for endergonic reactions
Do reactions with a negative ∆G or a positive ∆G occur spontaneously?
reactions with a negative ∆G occur spontaneously
Energy Coupling
how the energy released by ATP hydrolysis is used to perform work inside the cell
How do cells use ATP hydrolysis to power other chemical reactions?
cells couple the exergonic reaction of ATP hydrolysis with endergonic reactions (ATP coupling)
Describe the process of energy coupling
when ATP is hydrolyzed, the terminal phosphate group is transferred to a reactant molecule involved in an endergonic reaction. (remember phosphorylation)
Phosphroylation
the addition of a phosphoryl (PO3) group to a molecule
Spontaneous Chemical Reaction
occurs without any requirement for outside energy, but it may occur so slowly that it is imperceptible
Enzymes
biological catalysts; they increase the rate of chemical reactions by lowering the energy of activation (Ea)
What is the initial investment of energy for starting a reaction
ACTIVATION ENERGY
the energy required to contort the reactant molecules so the bonds can break
Enzymes regulate reactions for what reason
because most metabolic reactions need to surpass the activation energy before proceeding
Describe the Lock-and-key model
the shape of the substrate and the conformation of the active site are complimentary to another like a key in a lock or the missing piece to a puzzle
Describe the induced fit model
the enzyme undergoes a conformational change upon binding to substrate. The shape of the active site become complementary to the shape of a substrate only after the substrate binds to the enzyme
How does pH affect enzyme activity?
each enzyme has an optimal pH where it works at peak efficiency; rate of reaction decreases on wither side of the pH optimum
why are enzymes dependent on pH?
it is due to the ionizable amino acids; the pH changes modify the conformation of the protein and denatures the enzyme
How does temperature affect enzyme activity?
as the temperature rises, the rate of chemical reactions increase
if temperatures are too high, it will inactivate the enzymes because at high temps the enzyme molecules vibrate and twist so rapidly that some of the non-covalent bonds break
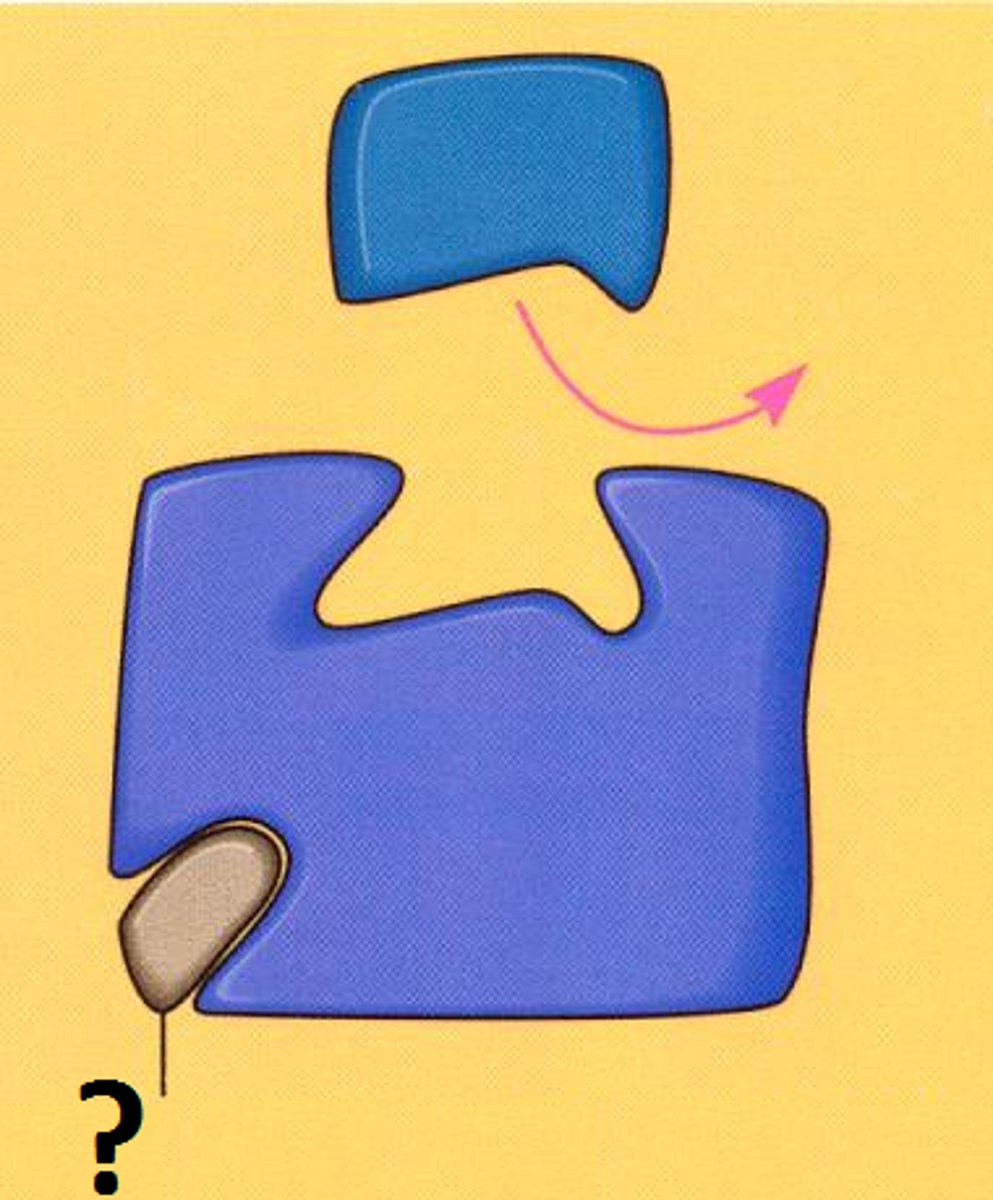
what is this an example of
Normal substrate binding to enzyme active site.
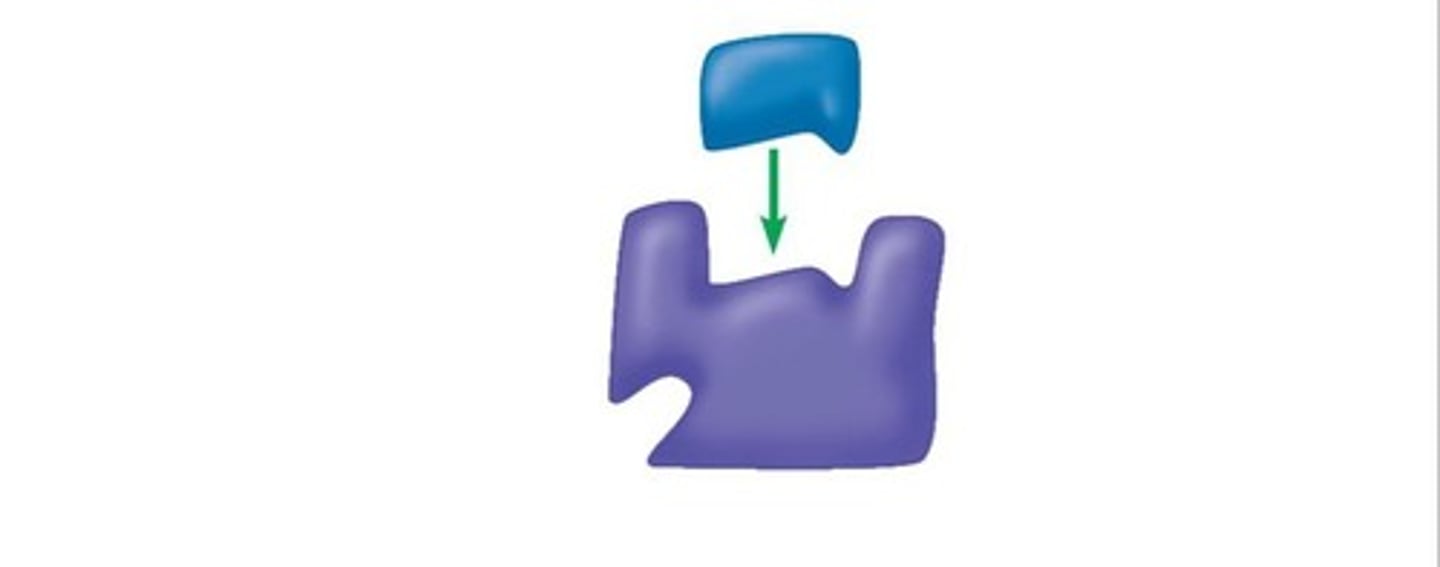
what is this an example of
a competitive inhibitor binds to the active site preventing substrate binding
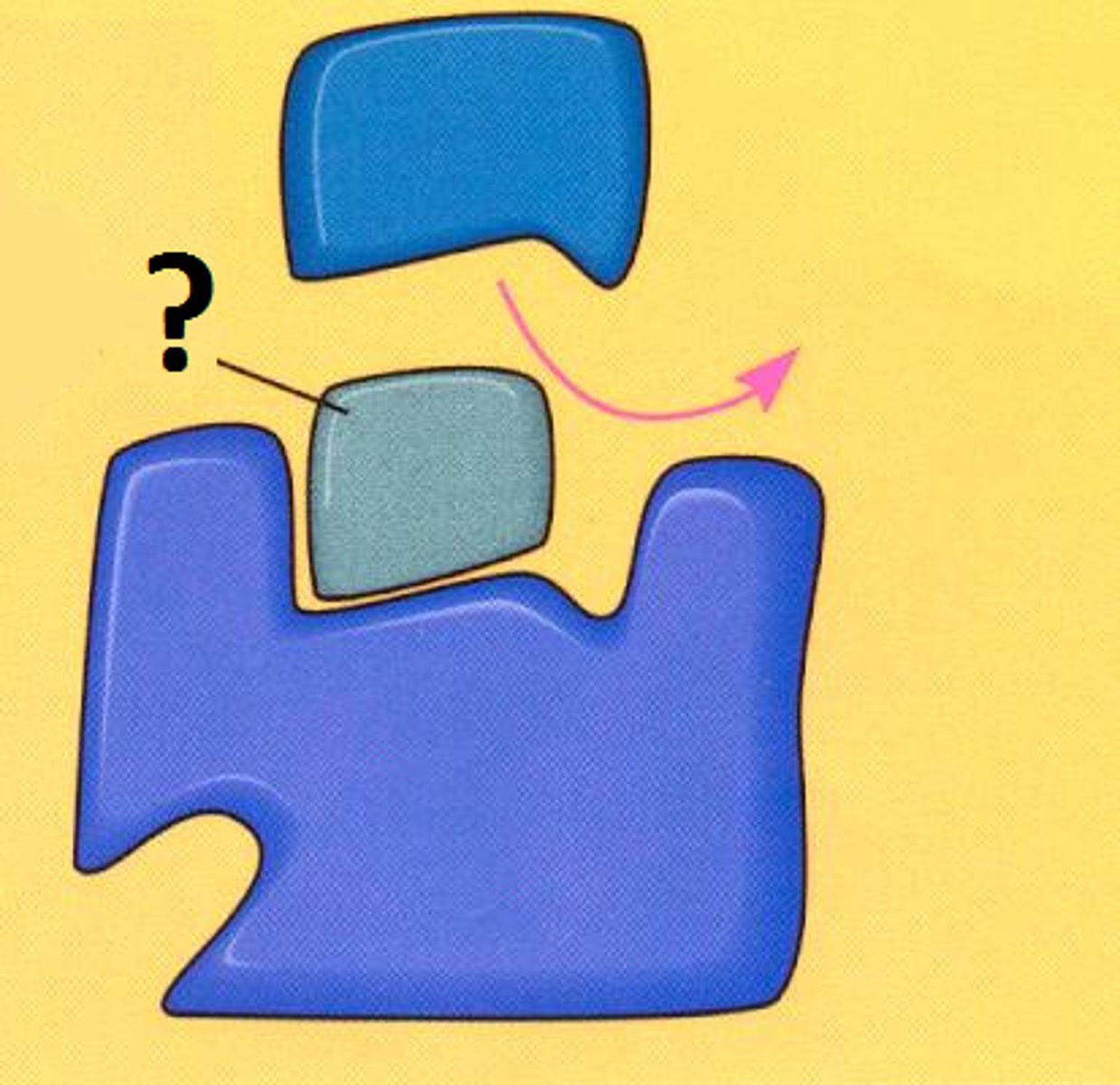
what is this an example of
a noncompetitive inhibitor
- binds at a site other than the active site, changing enzyme structure so that normal substrate binding cannot occur