C1 & C2: Atomic structure and the periodic table & Bonding, structure, and the properties of matter
1/51
Earn XP
Description and Tags
Topics covered: Atoms, elements, and compounds (White font) History of the atomic model (Yellow font) Atomic structure (Blue font) History of the periodic table (Red font) Groups 0, 1, and 7 (Purple font) Separation Techniques (Green font)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
52 Terms
Atom
The smallest part of an element, which cannot be broken down.
Compound
Contains two or more elements chemically combined in fixed proportions, and can be represented by the formulae of the atoms that make it up (e.g K2O - Potassium Oxide)
Naming a compound (metal and non-metal)
The metal name does not change
The non-metals name ends in ide
Sodium Sulfide, Potassium Oxide
Naming a compound (metal that reacts with ions that consist of two or more non-metal atoms covalently bonded together)
Metal name does not change
Non-metals name ends in ate if oxygen is present
E.g Sodium Carbonate, Potassium Nitrate
State Symbols
s = ?
l = ?
g = ?
aq = ?
s = solid, l = liquid, g = gas, aq = aqueous (dissolved in water)
Mixture
Consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance are unchanged.
Solvent
The liquid in which a solute dissolves
Solute
The substance that dissolves in a liquid to form a solution
Solution
Is the mixture formed when a solute has dissolved in a solvent
Soluble
Describes a substance that will dissolve
Insoluble
Describes a substance that will not dissolve
Filtration
Technique that separates substances which are insoluble in a solvent from those that are soluble.
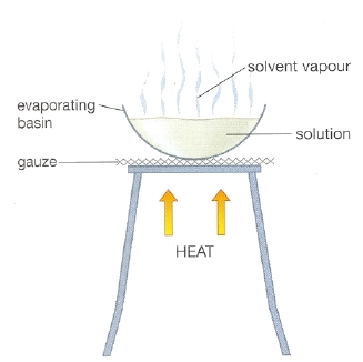
Crystallisation
Separates a soluble substance from a solvent by evaporation.
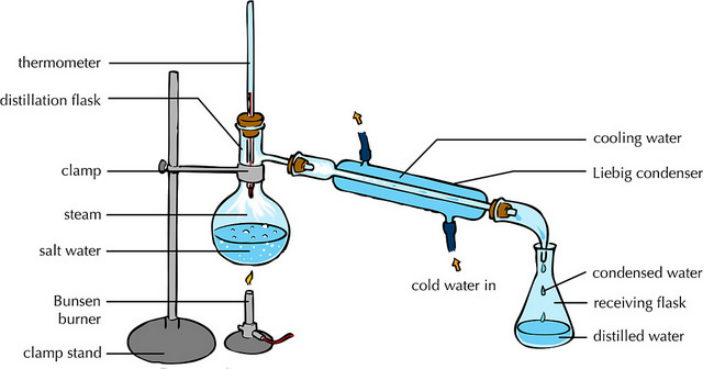
Simple distillation
Separates a solvent from a solution by evaporation followed by condensation
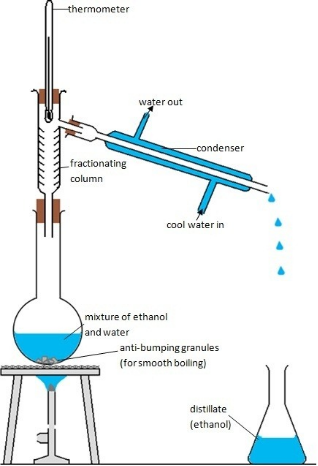
Fractional distillation
Separates a mixture into a number of different parts, called fractions. Substances with high boiling points condense at the bottom and substances with low boiling points condense at the top. Fractional distillation works because the different substances in the mixture have different boiling points.
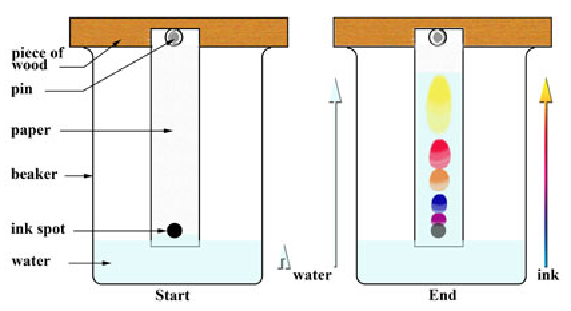
Chromatography
Separates small amounts of dissolved substances by running a solvent along absorbent paper.
John Dalton
Early 1800s. Before the discovery of electrons, John Dalton’s experiments led to the idea that atoms were tiny spheres that could not be divided.
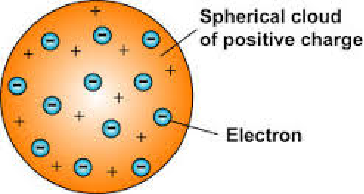
JJ Thompson
End of 1800s - The electron was discovered by JJ Thompson. Scientists believed that atoms were spheres of positive charge with negative charges spread throughout - the ‘plum pudding’ model.
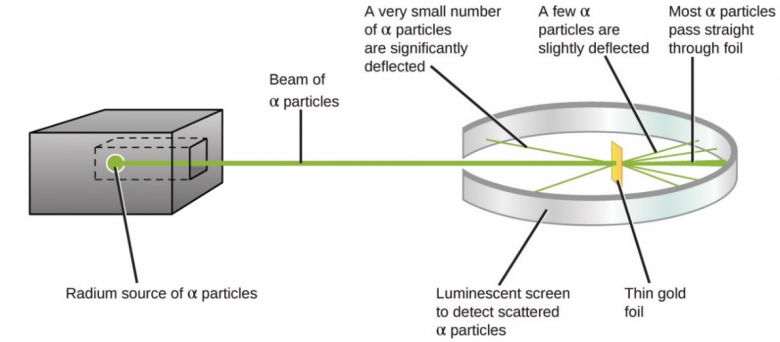
Ernest Rutherford
1908-1913 - Ernest Rutherford designed an experiment carried out by Geiger and Marsden. They fired alpha particles at a piece of very thin gold foil (only a few atoms thick) which scattered, leading to the conclusion that the mass of an atom was concentrated in a nucleus, which was charged. It proposed that electrons orbited around the nucleus.
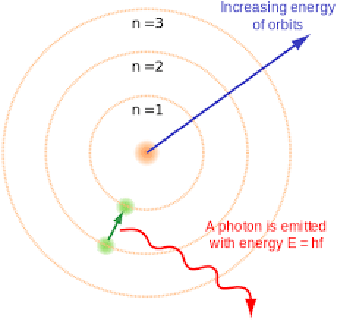
Niels Bohr
1914 - Niels Bohr noticed that the light given out when atoms were heated only had specific amounts of energy and he adapted the nuclear model by suggesting that electrons orbit the nucleus at specific distances in certain fixed energy levels (or shells). The energy must be given out when excited electrons fall from a high to low energy level. Protons were also discovered.
James Chadwick
1932 - Bombarded beryllium atoms with alpha particles. An unknown radiation was produced. Chadwick interpreted this radiation as being composed of particles with a neutral electrical charge and the approximate mass of a proton. This particle became known as the neutron.
Subatomic particles properties
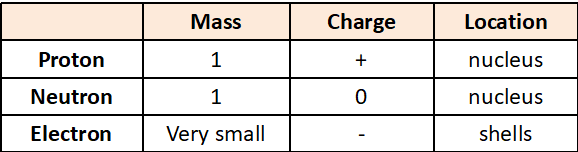
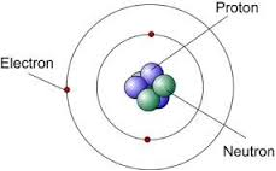
Atomic Number
The number of protons in an atom.
Mass Number
Number of Protons and Neutrons in an atom
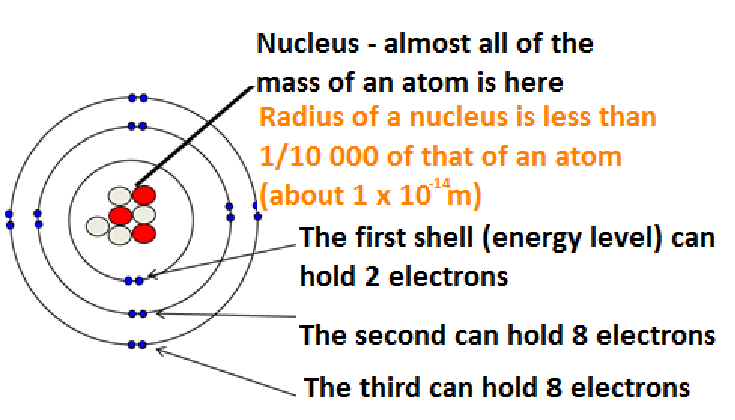
Shell Structure
1st shell - 2 electrons
2nd shell - 8 electrons
3rd shell onwards - 8 electrons
What happens to the charge of an atom if it loses/gains electrons?
If it loses an electron, it gains a positive charge. If it gains an electron, it gains a negative charge.
Isotope
Atoms of the same element that have different numbers of neutrons.
Relative Atomic mass
The average value that takes into account the abundance of the isotopes of the element. Samples of different isotopes of an element have different physical properties (e.g density) but always have the same chemical properties.
Relative Atomic mass equation
(% of isotope 1 x mass of isotope 1) + (% of isotope 2 x mass of isotope 2) / 100
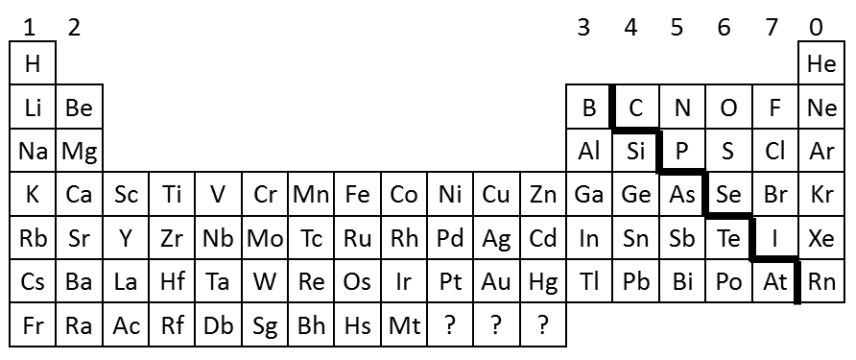
Periods + Groups
The rows in the table are called periods. Elements in the same period have the same number of shells.
The columns are called groups. Elements in the same groups have similar properties, and have the same number of electrons in their outer shell.
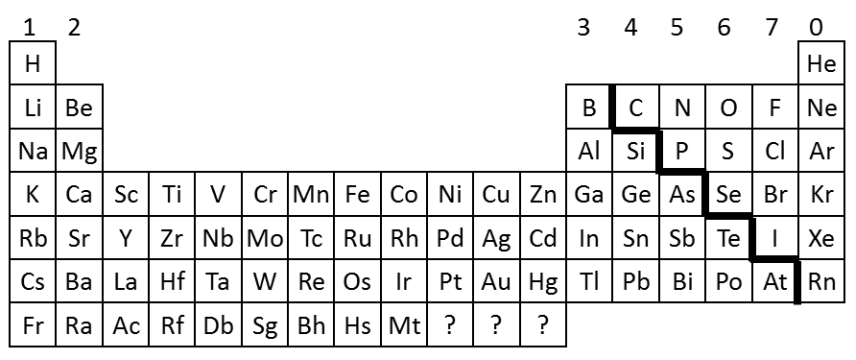
1808
John Dalton published a table of elements that were arranged in order of their atomic weights, which had been measured in various chemical reactions

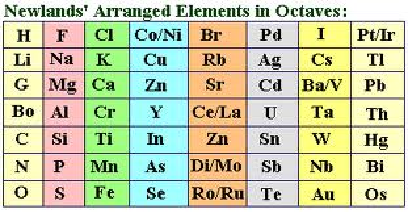
1864
John Newlands published the law of octaves. However the table was incomplete and elements were placed in inappropriate groups
1869
Dmitri Mendeleev overcame Dalton’s problem by leaving gaps for the elements that he thought had not been discovered and in some places changed the order based on atomic weight (e.g. Argon and Potassium). Elements with properties predicted by Mendeleev were eventually discovered.
Metal Properties
Shiny
Mostly solid
Dense
Strong
Malleable
Good conductor of heat
Good conductor of electricity
Non Metal Properties
Dull
Low density
Weak
Brittle
Poor heat conductor
Poor electrical conductor
Ions in reference to metals and non metals
Non-metals tend to form negative ions, while metals tend to form positive ions.
Metals and non metals in the periodic table
Metals are found towards the left and bottom. Non metals are found towards the top and right.
Noble Gases
Elements in Group 0 of the periodic table are called the noble gases. They are unreactive because their atoms have stable arrangements of electrons. The atoms have eight electrons in their outermost shell, apart from helium, which has just two, but still has a complete outer shell.
The stable electronic structure explains why they exist as single atoms, they have no tendency to react to form molecules.
The boiling points of the noble gases get higher going down the group. For example helium boils at -269 °C and radon boils at -62°C.
Alkali Metals
The alkali metals are very reactive. They need to be stored under oil to prevent them reacting with oxygen and water vapour in the air. The alkali metals have low densities. The metals are very soft and can be cut with a knife. They also have low melting and boiling points, which decrease as you go down the group, and as you go down, reactivity increases.
The properties are due to all the atoms having just one electron in their outermost shell. They only need to lose one electron to get the stable electronic structure of a noble gas.
Alkali Metals → Shielding
The atoms get larger as you go down, so the single electron in the outermost shell (highest energy level) is attracted less strongly to the positive nucleus. The electrostatic attraction with the nucleus gets weaker because the distance between the outer electron and the nucleus increases. Also the outer electron experiences a shielding effect from the inner electrons, reducing the attraction between the oppositely charged outer electron and the nucleus.
Halogens
The halogens are a group of toxic non-metals that have coloured vapours. They have low melting and boiling points, which increase as you go down the group. They are poor conductors of heat and electricity.
As elements, the halogens exist as molecules made up of pairs of atoms. These are called diatomic molecules (bonded covalently) F2, Cl2, Br2, I2 and At2. The halogens have seven electrons in their outermost shell and need to gain one electron to achieve the stable electronic structure of a noble gas. When they react with non metals, they are joined together by a covalent bond.
Halogens → Shielding
When Group 7 elements react, the atoms gain an electron in their outermost shell. Going down the group, the outermost shell’s electrons get further away from the attractive force of the nucleus, so it is harder to attract and gain an extra electron. The outer shell will also be shielded by more inner shells of electrons, again reducing the electrostatic attraction of the nucleus for an incoming electron. As you go down the group, reactivity decreases.
The Transition metals
The transition metals are located between group 2 and group 3.
The transition metals have:
High melting points, high boiling points and high densities.
They are:
Shiny when polished
Malleable – can be hammered into a shape
Strong – don’t break easily when a force is applied
Sonorous – makes a ringing sound when hit
Ductile – can be stretched into wires
Conductors of electricity and heat
As you go across the group, reactivity generally decreases.
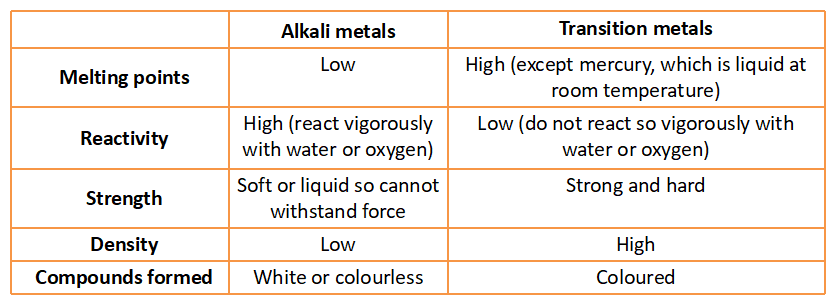
Difference in properties between Transition metals and Alkali metals.
Structure of an atom
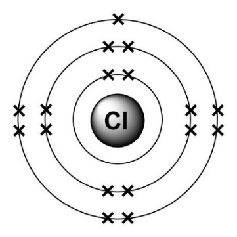
Electronic configuration of Chlorine?
2, 8, 7
When adding a sodium hydroxide solution to an Aluminium (Al3+) solution, what colour precipitate will be formed?
White
When adding a sodium hydroxide solution to a Calcium (Ca2+) solution, what colour precipitate will be formed?
White
When adding a sodium hydroxide solution to a Magnesium (Mg2+) solution, what colour precipitate will be formed?
White
When adding a sodium hydroxide solution to a Copper II (Cu2+) solution, what colour precipitate will be formed?
Blue
When adding a sodium hydroxide solution to an Iron II (Fe2+) solution, what colour precipitate will be formed?
Green
When adding a sodium hydroxide solution to an Iron III (Fe3+) solution, what colour precipitate will be formed?
Brown