Drugs and Behavior Exam 1
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
What is pharmacodynamics?
the study of how a drug affects the body
What is pharmacokinetics?
study of the movement of drugs within the body
What is pharmogenomics?
study of how an individual's genes affect their response to medications
What is pharmacogenetics?
the study of how a person's genes influence their unique response to medications
What are instrumental drugs?
drugs that are used to address a specific purpose (prescriptions)
What are recreational drugs?
drugs that are used to experience its effects and not used for treatment (includes drugs that are not your prescription)
What are misuse drugs?
these are drugs that are intended for instrumental purposes but are used recreationally instead
What are brand name drugs?
a trademark name that a company provides for a drug
What are generic name drugs?
nonproprietary name that indicates the classification for a drug and distinguishes a drug from others in the same class
What are chemical name drugs?
details a drugs chemical structure
What are street name drugs?
an alternative name applied to a recreational or abused substance
How does drug amount/body weight effect dose?
If a person weights more, they may need to take a higher dose compared to a person who weighs less. dosage is the ratio of a drug per an organisms body weight
What is the dose effect curve?
depicts the magnitude of a drug effect by dose. can show the potency of a drug depending on dose and percent effect
What is chlorpromazine and describe its impact.
Chlorpromazine is the first widely used antipsychotic medication, introduced in the early 1950s. Originally developed as an antihistamine, it was found to calm agitation and reduce psychotic symptoms like hallucinations and delusions.
Its impact on psychopharmacology was revolutionary:
It marked the beginning of modern psychiatric drug treatment, shifting care away from asylums toward outpatient management.
It provided the first effective medical alternative to invasive treatments like lobotomy or electroshock therapy.
It launched the development of the phenothiazine class of drugs and paved the way for other antipsychotics, fundamentally shaping psychiatry as a medical discipline.
When chlorpromazine was introduced, no one knew exactly why it worked. Later research showed that it blocks dopamine receptors (especially D2 receptors) in the brain. This was groundbreaking because it:
Provided the first strong evidence that mental illness could be linked to neurotransmitters rather than purely psychological or moral causes.
Led to the dopamine hypothesis of schizophrenia, suggesting that overactive dopamine signaling contributes to psychosis.
Opened the door for the study of other neurotransmitters (like serotonin, norepinephrine, and GABA) in mood and anxiety disorders.
In short, chlorpromazine didn’t just treat symptoms—it reshaped psychiatry into a neuroscience-based field, showing that brain chemistry could be targeted with drugs to manage mental illness.
What system is responsible for delivering voluntary motor signals from the CNS to muscles and for conveying sensory information from the body to CNS?
somatic nervous system
What system control involuntary movements like breathing, heart rate, etc?
autonamic nervous system
What system prepares the body for rigorous activity and other involuntary functions?
sympathetic nervous system
What system is dominant during relaxed states and decreases heartbeat and stimulates digestion?
parasympathetic nervous system
What system controls digestion via communication within its systems and the CNS?
enteric nervous system
What is the CNS?
brain and spinal cord
What is the PNS?
the rest of the body (not including brain and spinal cord)
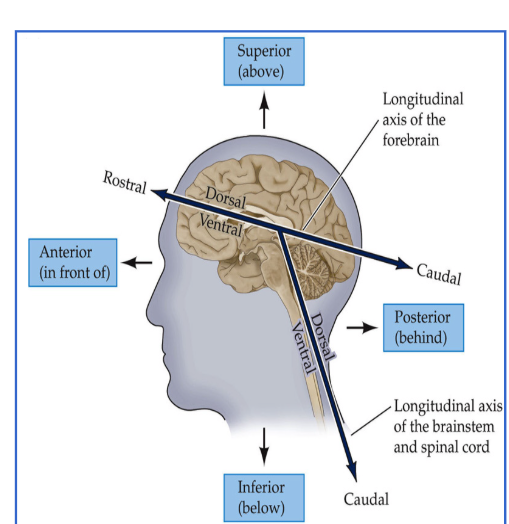
Know brain directions.
What is the limbic system?
a group of interconnected brain structures that help regulate your emotions and behavior. This system works together with other brain regions by processing your memory, thoughts and motivations, then tell your body how to respond.
What is basal ganglia?
a cluster of nuclei found deep to the neocortex of the brain. It has a multitude of functions associated with reward and cognition but is primarily involved in motor control
What is acetylcholine?
Plays a role in the central nervous system, autonomic nervous
system, and peripheral nervous system.
What is serotonin?
An inhibitory neurotransmitter that affects mood and feelings of well-
being.
What is dopamine?
involved in reward and pleasure
What is GABA?
An inhibitory neurotransmitter that reduces
neural activity
What is glutamate?
An excitatory neurotransmitter that enhances neural communication.
What is epinephrine?
Involved in the "fight or flight" response.
What is norepinephrine?
affects attention and alertness
What neurotransmitter modifies learning?
acetylcholine
What neurotransmitter modifies mood?
seratonin
What neurotransmitter modifies pleasure?
dopamine
What neurotransmitter modifies calming?
GABA
What neurotransmitter modifies memory?
glutamate
What neurotransmitter modifies survival?
adrenaline (epinephrine)
What neurotransmitter modifies concentration?
noardrenaline
What neurotransmitter modifies euphoria?
endorphins
What neurotransmitter modifies attention and alertness?
norepinephrine
Why are animals used in drug trials?
they are mainly used to determine if a drug is safe by testing dosages, chemistry and its effects in the body
Explain the Oxycontin and Prairie Vols case study.
The Oxycontin and prairie vole case study examined how oxytocin, sometimes nicknamed the “bonding hormone,” influences social attachment. Prairie voles, which naturally form lifelong pair bonds, were given oxytocin, which enhanced bonding behaviors, while blocking oxytocin receptors reduced or eliminated pair bonding. From this, researchers concluded that oxytocin plays a critical role in forming and maintaining social attachments. The key takeaway is that biochemistry, specifically oxytocin signaling, underlies complex social behaviors like trust, bonding, and attachment, highlighting how neurochemistry can shape relationships.
In humans, researchers believe oxytocin has similar effects on trust, intimacy, and social bonding. Studies show it’s released during hugging, sex, childbirth, and breastfeeding—situations tied to closeness and connection. While it doesn’t “cause love,” oxytocin seems to reinforce feelings of attachment and trust, helping build long-term bonds between partners, parents and children, and even within groups. The key idea carried over from the prairie vole studies is that our social relationships are strongly influenced by neurochemistry, not just by choice or environment.
Limitations:
Species differences: Prairie voles are unusually monogamous for rodents, so their oxytocin system may not fully reflect human bonding, which is more complex and variable.
Oversimplification: While oxytocin plays a role, human attachment also involves dopamine, vasopressin, social learning, and cultural factors—so it’s not just a “love hormone.”
Context matters: In humans, oxytocin can increase trust and bonding, but in some situations, it may also heighten in-group favoritism or even jealousy, showing its effects aren’t universally positive.
What are chimera?
an organism made up of cells from two or more genetically distinct individuals.
What does the Center for Drug Evaluation and Research (CDER) do?
ensures that safe and effective drugs are available to
improve the health of the people in the United States
What does the FDA do?
approve and regulate drugs by ensuring long efficacy and aproove drug and study of it.
Is a drug prefer reviewed after initial FDA approval?
No, if anything occurs corporation funds maintain and address grievances
What is phase 1 of the 4 stages of drug approval?
focuses on the safety of the drug using 20-80 healthy volunteers to see what the drug does to the body. (pharmokinetics)
What is phase 2 of the 4 stages of drug approval?
focuses on effectiveness by recruiting 40 to 300 patients to determine the effectiveness of the drug on sick people (pharmokinetics).
What is phase 3 of the 4 stages of drug approval?
paitnets are monitored in hospitals to determine effectiveness of drug if evidence of effectiveness is shown in phase 2. Side effects are also reported here (pharmodynamics)
What is phase 4 of the 4 stages of drug approval?
when researchers gather additional information such as a products safety, efficacy or optimal use after approval. this phase is not required occurs post NDA approval (pharmogenomics)
What does a drug do to the body?
disrupts, alters. or interacts with communication processes in the brain (if its a pshycotropic drug) or can cause change in behavior
Step by step process of FDA drug approval
Discovery & preclinical testing — lab and animal studies to show proof-of-concept, basic pharmacology, toxicology, and to develop manufacturing methods (CMC = chemistry, manufacturing, controls). Good Laboratory Practice (GLP) studies and scalable manufacturing data are generated so humans can be safely dosed.
FDAApprovalProcess
IND (Investigational New Drug) application — the drug sponsor (company or investigator) files an IND with the FDA to request permission to start clinical trials in humans. An IND contains: animal pharmacology/toxicology, CMC, proposed clinical protocols, investigator’s brochure, and safety information. The FDA reviews for safety and can place a clinical hold if concerns exist.
FDAApprovalProcess
Clinical trials — Phase I → III
Phase I: small studies (often healthy volunteers, sometimes patients) focused on safety, tolerability, pharmacokinetics and dose-finding (dose-escalation designs; oncology differs). Institutional Review Boards (IRBs) and informed consent are required; sponsors often use Data & Safety Monitoring Boards (DSMBs) for higher-risk studies.
FDAApprovalProcess
Phase II: proof-of-concept and dose-ranging trials in patients to evaluate efficacy signals and continue safety assessment; refines endpoints and trial design.
FDAApprovalProcess
Phase III: large, randomized, controlled (pivotal) trials intended to provide the definitive evidence of benefit/risk; results are the primary basis for regulatory approval. Trials must follow Good Clinical Practice (GCP).
FDAApprovalProcess
Regulatory filing — NDA / BLA / alternatives
NDA (New Drug Application) for most small-molecule drugs (505(b)(1) = full; 505(b)(2) = relies partly on existing literature/data).
BLA (Biologics License Application) for biologics (e.g., monoclonal antibodies, vaccines).
ANDA (Abbreviated New Drug Application) for generics (bioequivalence rather than de novo clinical efficacy).
Filings include integrated clinical summaries, full datasets, nonclinical data, CMC, proposed labeling, safety analyses, and risk-management plans. Fees and review timelines are governed by PDUFA (user-fee) agreements.
FDAApprovalProcess
FDA filing decision & multidisciplinary review — after intake the FDA “files” the application (accepts it for review) and assigns multidisciplinary review teams (clinical, statistical, pharmacology/toxicology, CMC, biostatistics, pharmacovigilance). The agency may request advisory committee input (external experts) for complex or controversial decisions; advisory votes are advisory, not binding.
FDAApprovalProcess
Inspections & labeling / risk-management — FDA conducts pre-approval facility inspections (GMP compliance) of manufacturing sites and inspects clinical sites if needed. Label (prescribing information) and post-market risk-management (e.g., REMS = Risk Evaluation and Mitigation Strategy) are negotiated before approval.
FDAApprovalProcess
Regulatory decision — outcomes include: approval (with final labeling), approval with post-marketing conditions, Complete Response Letter (CRL) requiring more data/changes, or approvable/deniable responses. If approved, the product can be marketed per the approved label.
FDAApprovalProcess
Post-marketing / Phase IV obligations — FDA monitors safety through mandatory reports (e.g., adverse event reporting), sponsor-required post-marketing studies (PMRs) or commitments (PMCs), periodic safety updates, and active surveillance (FAERS/MedWatch). Safety signals can lead to label changes, REMS, additional trials, restricted distribution, or withdrawal.
FDAApprovalProcess
Special pathways & designations — programs that change interaction and timing include: Orphan Drug designation (incentives for rare diseases), Fast Track, Breakthrough Therapy, Priority Review, and Accelerated Approval (allows approval based on surrogate endpoints with confirmatory trials required). Emergency Use Authorization (EUA) is a separate, temporary pathway used in public-health emergencies.
FDAApprovalProcess
Other actors & compliance — besides the FDA centers (primarily CDER — Center for Drug Evaluation and Research — for drugs; CBER — Center for Biologics Evaluation and Research — for biologics), other FDA offices participate (Office of New Drugs, Office of Compliance, Office of Regulatory Affairs for inspections). Sponsors, clinical investigators, IRBs, contract research organizations (CROs), and external DSMBs are all essential players.
Approval Proccess simplfied
The FDA drug approval process begins with preclinical lab and animal testing, followed by an IND application to start human trials. Clinical trials progress through Phase I (safety/dosing), Phase II (efficacy and safety), and Phase III (large pivotal trials) under IRB and GCP oversight. If successful, sponsors file an NDA (for drugs) or BLA (for biologics), which undergoes FDA review by multidisciplinary teams, advisory committee input if needed, and facility inspections. The FDA then decides on approval, labeling, and any risk management plans (like REMS). After approval, Phase IV studies and safety monitoring continue through FAERS and MedWatch. Special pathways—such as Fast Track, Breakthrough, Priority Review, and Accelerated Approval—can speed access for serious conditions. The process is evidence-driven, ensuring drugs are safe, effective, and manufactured to quality standards before reaching patients.
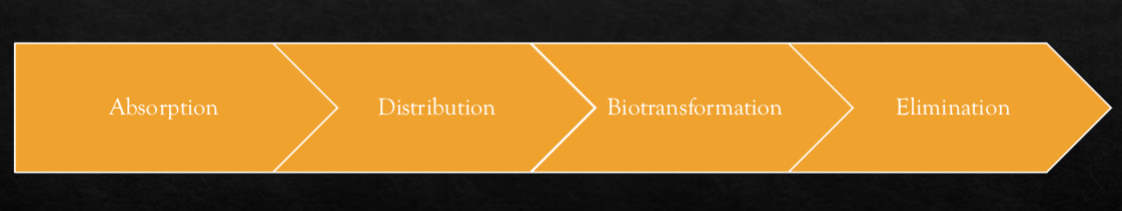
Explain elimination in relation to the pharmakinetics of drugs.
Elimination is the process of how a drug leaves the body.
long acting drugs have a steady release
Dose dumping is when a controlled-release drug releases too quickly, overwhelming the body and potentially causing harm. (microplastics from drug dissolve in stomach acid and cause problems)
2 main elimination mechanisms:
Metabolism – The liver transforms drugs into more water-soluble compounds (often inactive) so they can be excreted.
Excretion – The kidneys usually excrete drugs in urine, but drugs can also be eliminated via bile, feces, sweat, or breath.
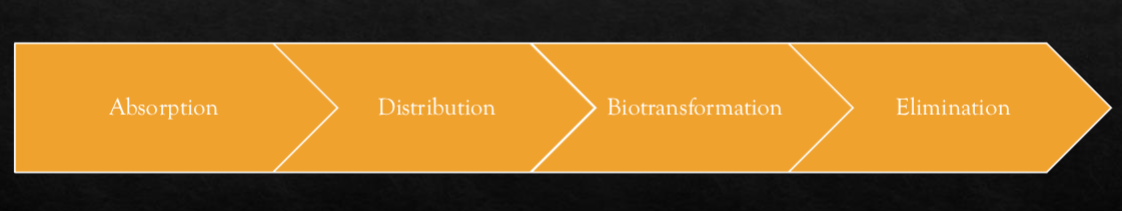
Explain biotransformation in relation to the pharmakinetics of drugs.
converts drug into metabolites (chemical product after biotransformation)
Phase 1 biotransformation (if drug is water solubkle it gets kicked out here)
Phase 2 biotransformation (if drug is fat soluble, the body goes though extra processes so the drug can be excreted from body)
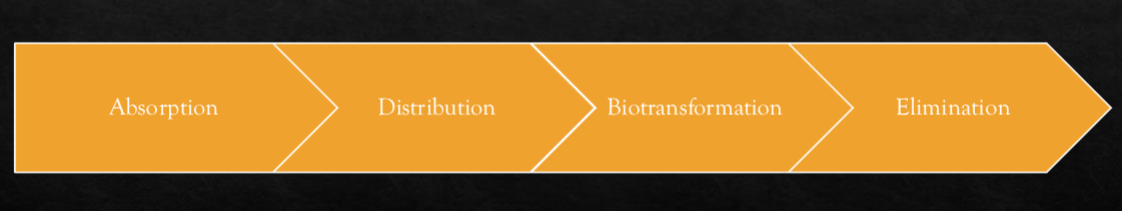
Explain distribution in relation to the pharmakinetics of drugs.
the passage of drug from bloodstream to body sites
bioavalibility (how much of a drug gets distributed once absorbed)
there is a reversible transfer of a drug between blood and tissue
drugs travel slower in fat
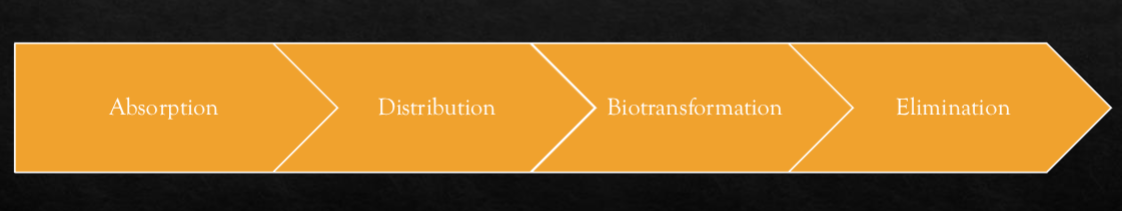
Explain absorption in relation to the pharmakinetics of drugs.
diffuses ionized (does well in basic environments) and nonionized (does well in acidic environments) molecules (fart in a room example)
each drug in your body diffuses differently based on how its administered
liberation (when the drug is dissolved impacted by the type of encapsulation
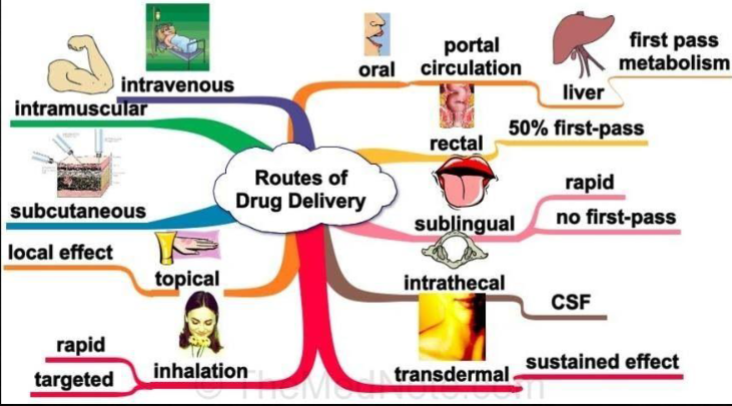
Routes of drug delivery.
What are gap junctions?
not flexible, cant amplify or diminish signal from one neuron to the next, of ten found in inter neurons where synchrony us needed for collective firing one time
What is binding affinity?
how strongly a drug BINDS to its receptor (binds tightly at low concentrations(high affinity) and binds weakly at high concentrations (low affinity))
What is receptor efficacy?
the ability if a drug, once bound, to activate the receptor and produce a biological response
T or F: Synthesis of GABA makes glutamate.
T
Describe deinstitutionalization as it relates to the pharmaceutical industry.
-treating mental illness w/ sedation
Introduction of psychiatric drugs
The development of drugs like chlorpromazine (the first antipsychotic) in the 1950s allowed psychiatrists to manage symptoms with medication rather than only sedation.
Later, clozapine (an atypical antipsychotic) provided a treatment for patients resistant to older drugs, reducing severe psychotic symptoms and the need for institutionalization.
Impact on deinstitutionalization
Medications made it possible for patients to live outside hospitals while still managing their symptoms.
This reduced the need for long-term confinement and shifted care to outpatient and community settings.
Sedation vs. targeted treatment
Early treatments often relied on sedating patients to control behavior.
Modern psychopharmacology focuses on targeting neurotransmitters (dopamine, serotonin) to treat symptoms rather than just calming patients.
Where is glutamate usually found?
Basal ganglia
What are neurotropic viruses?
viruses that specifically target the nervous system, meaning they can infect nerve cells (neurons) or other cells in the brain, spinal cord, or peripheral nerves.
What is the first pass effect?
is the liver “filter” that reduces the amount of orally taken drug that actually reaches the bloodstream.
What process converts drugs into metabolites?
biotransformation
What does 120/70 mean in relation to psychiatry and psychopharmacology?
the age of the fields in years within Western medicine
What are agonist?
drugs that occupy receptors and activate them
What are antagonists?
drugs that occupy receptors but do not activate them, they also block receptor activation by agonists
What brain region includes the basal ganglia and thalamus. WHat relevance does this have to neurotransmitters?
Basal ganglia + thalamus = subcortical brain region
Psychotropic drugs target this region to adjust neurotransmitter activity These areas are important in mental illness because they regulate mood, reward, motivation, and behavior.
Key neurotransmitters: dopamine (movement, reward) and serotonin (mood, cognition)
Dopamine: Highly active in the basal ganglia; affects movement, reward, and motivation. Many antipsychotics block or modulate dopamine.
Serotonin: Modulates mood, anxiety, and cognition; many antidepressants (SSRIs) target serotonin pathways that influence subcortical regions.
What are the three phases of action potential?
depolarization, repolarization and hyperpolarization
What type of communication does action potentials create and how does it relates to drugs?
electric communication
drugs can modulate how these signals are sent or received by:
Changing neurotransmitter release (Some drugs increase or decrease neurotransmitter release, which affects the strength or frequency of action potentials)
Blocking or activating receptors (mimic or block neurotransmitters at synapses)
Altering ion channel activity
Modifying neurotransmitter clearance (By preventing neurotransmitter reuptake or breakdown, drugs prolong the effect of neurotransmitters, indirectly influencing action potential signaling.)
What are ionotropic receptors?
receptors that are fast and ion-channel based
What is the blood brain barrier?
protective barrier between the blood and the brain that controls what substances can enter the brain from the bloodstream.

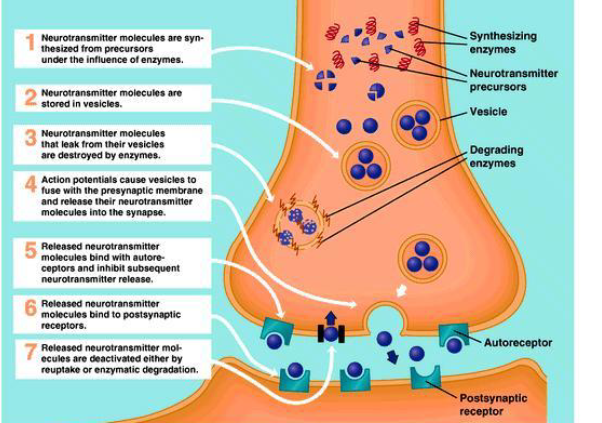
Define and name 3 of 7 major processes in neurotransmission that drugs can alter.
What is a half-life?
the amount of time it takes for half of a substance—like a drug—to be eliminated from the body.
What is the DEA?
drug enforcement agency
What are schedule I drugs?
highly addictive drug with no approved medical use
What are schedule II drugs?
high potential abuse but has medical use, has either physical OR psychological dependence
What are schedule III drugs?
abuse potential is small, moderate to low potential for physical and psych dependence
What are schedule 4 drugs?
low abuse and low dependence drugs that needs a script
What are schedule 5 drugs?
low abuse and low dependence drugs that are received over the counter