intro to aromatic chem ...
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
draw Kekule’s structure of benzene:
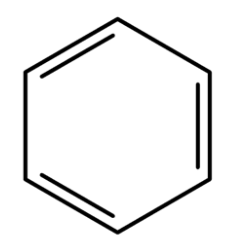
give 3 issues with Kekule’s structure of benzene:
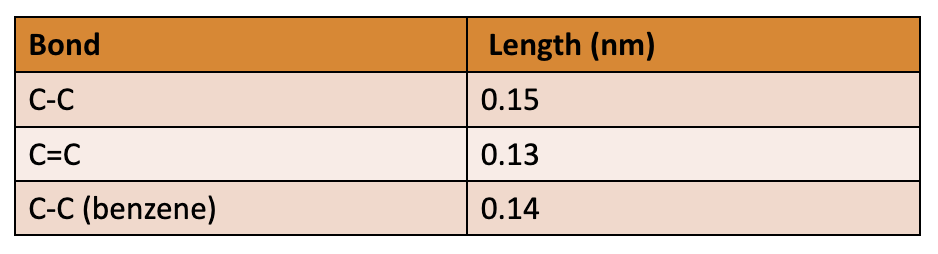
C-C bonds in benzene are all the same length (C-C and C=C bonds are different lengths)
benzene does not undergo addition reactions readily
ΔH hydrogenation about 152 kJ mol-1 less exo than expected
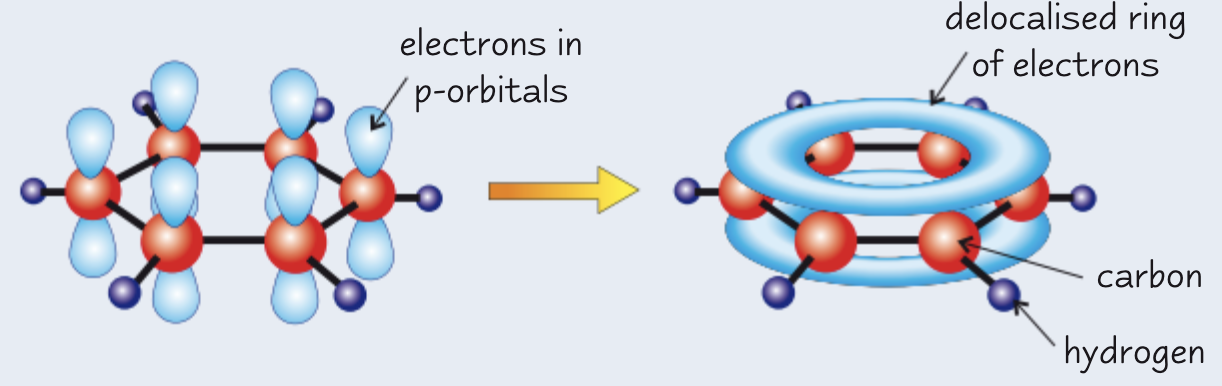
describe the (actual) structure of benzene:
each C has 3 covalent bonds
spare e- in a p orbital overlap to form a π cloud and are delocalised
planar
6 C ring with 120o bond angle
C-C bonds = in length, length in between lengths of typical C-C and C=C bond
why is benzene more stable than the theoretical molecule cyclohexa-1,3,5-triene?
benzene has delocalisation of p e-
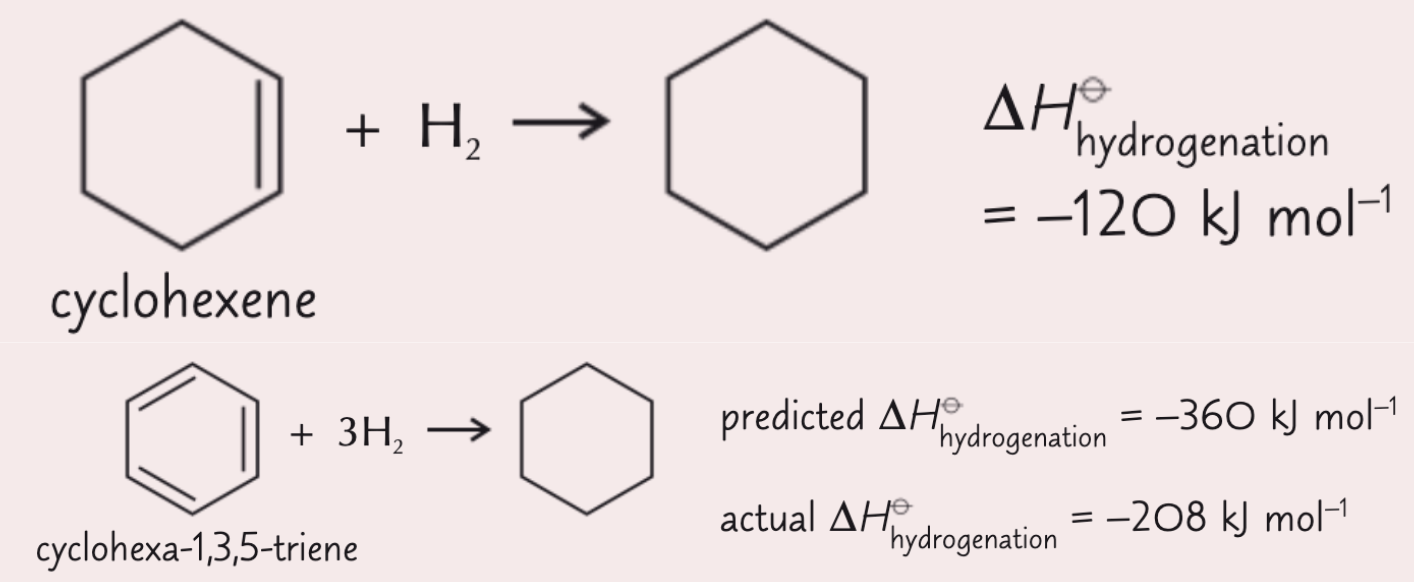
how can we use thermochemical evidence from enthalpies of hydrogenation to account for the extra stability of benzene?
enthalpy of hydrogenation of cyclohexene + H2 → cyclohexane = -120 kJ mol-1
so if the structure of the hypothetical triene was accurate, we would expect triene + 3H2 → cyclohexane = -360 kJ mol-1
but the real value for the enthalpy of hydrogenation is 208 kJ mol-1 which is 152 kJ mol-1 less exothermic than we might expect ∴ benzene is 152 kJ mol-1 more stable than the hypothetical triene molecule
this extra stability is due to the delocalisation of p e- and is known as the delocalisation stability
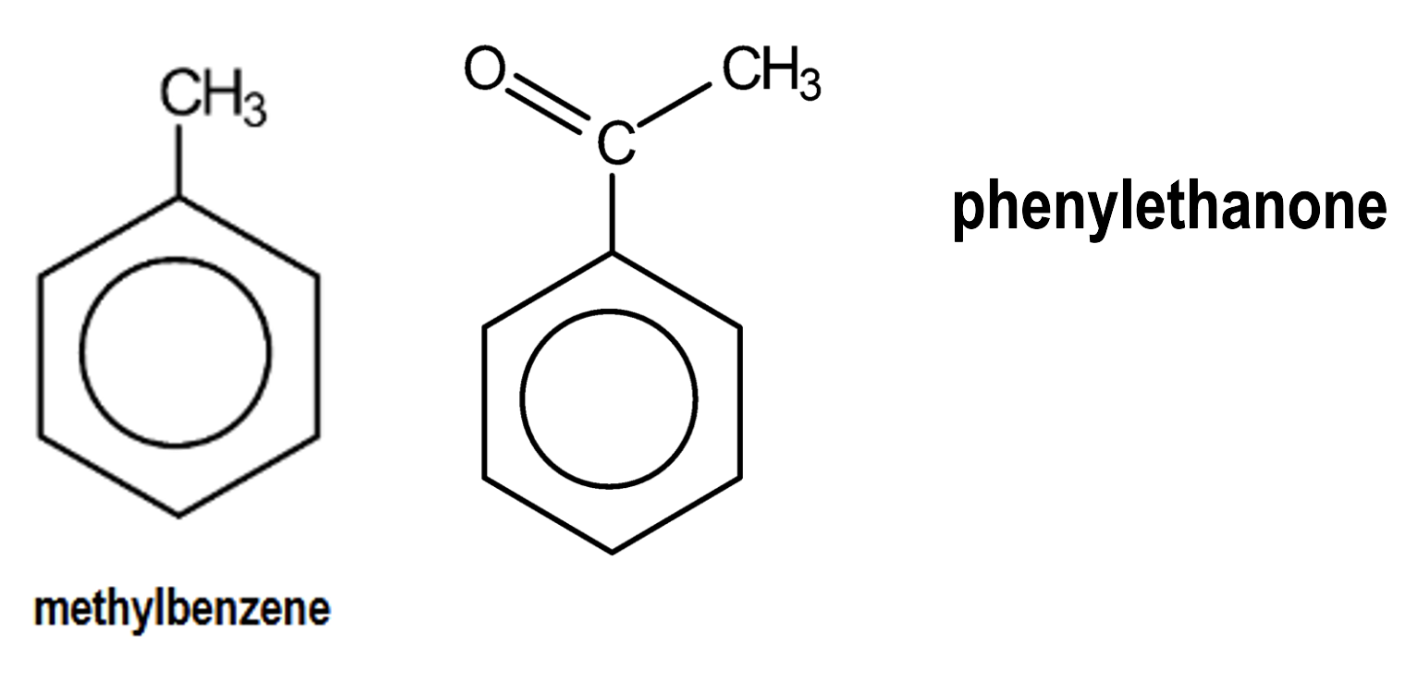
what are the prefixes/suffixes used to name aromatic compounds?
benzene (suffix) - e.g. methylbenzene
phenyl (prefix) - e.g. phenylethanone