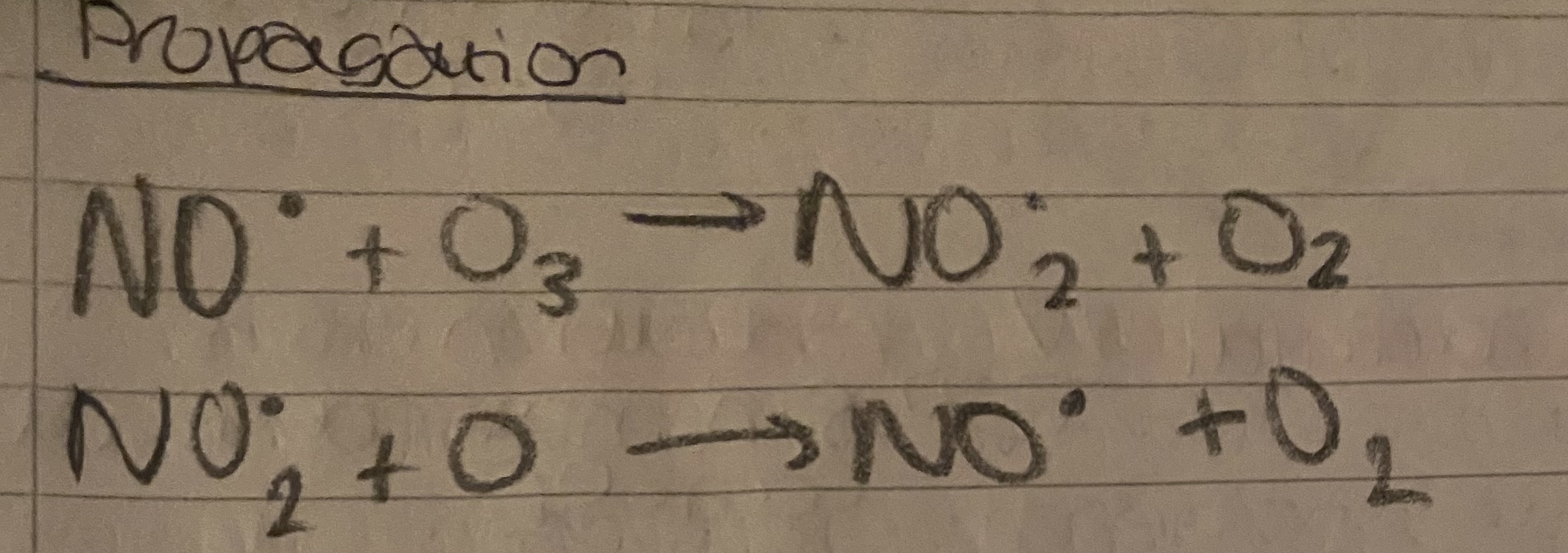Ozone
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What does ozone look like bonded?
.
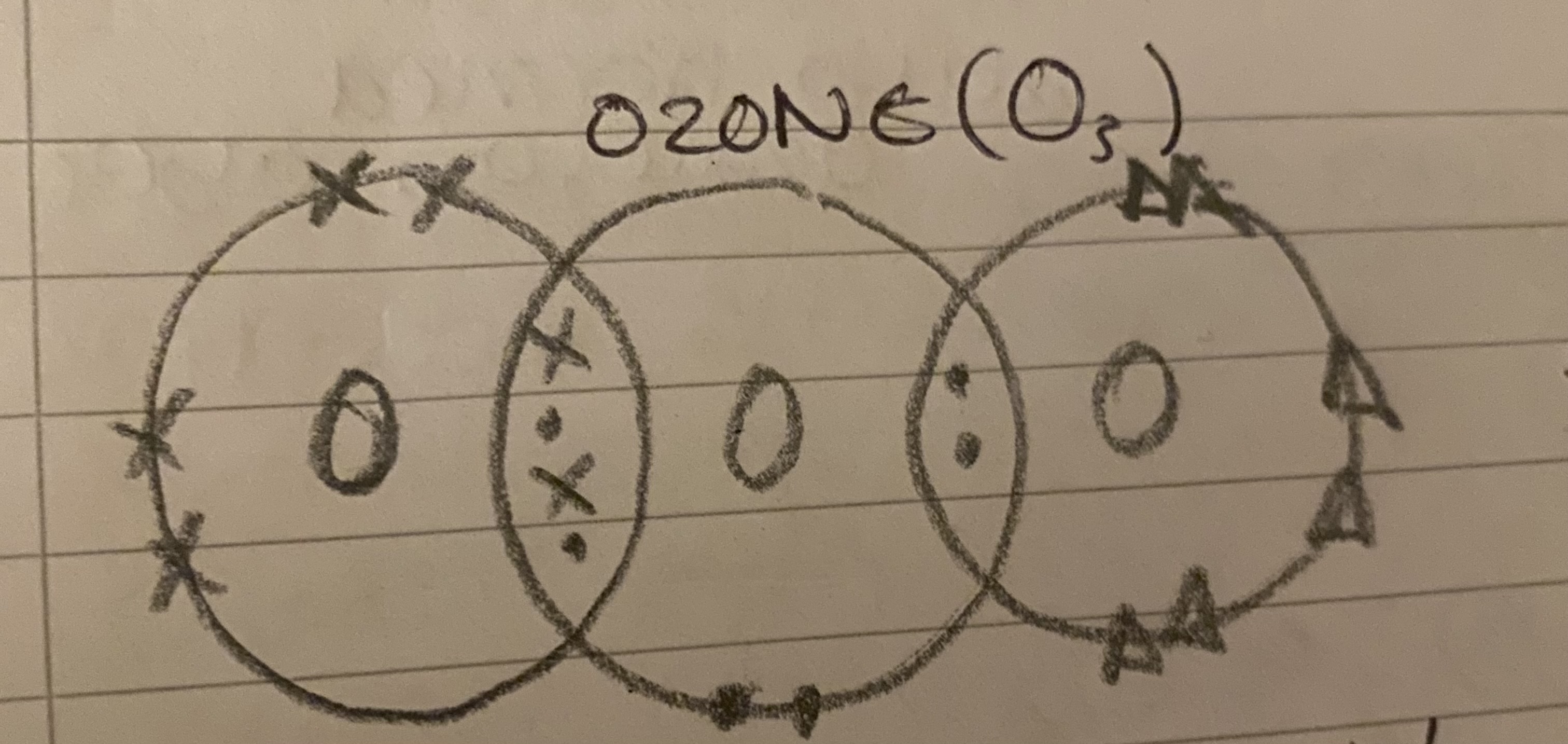
Why is O3 important?
It is in the ozone layer which removes harmful UV. This protects us from radiation due to the sun rays, which can cause cancer.
Is ozone in an equilibrium?
The rate at which it forms is the rate at which it breaks.
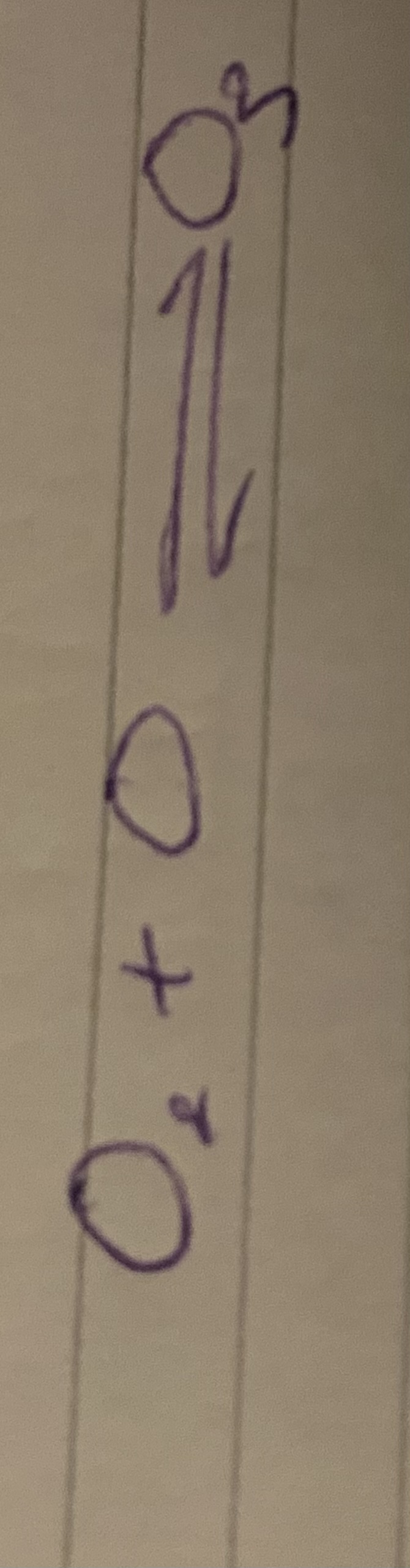
What harms the ozone layer?
CFC’s (chloroflurocarbons)
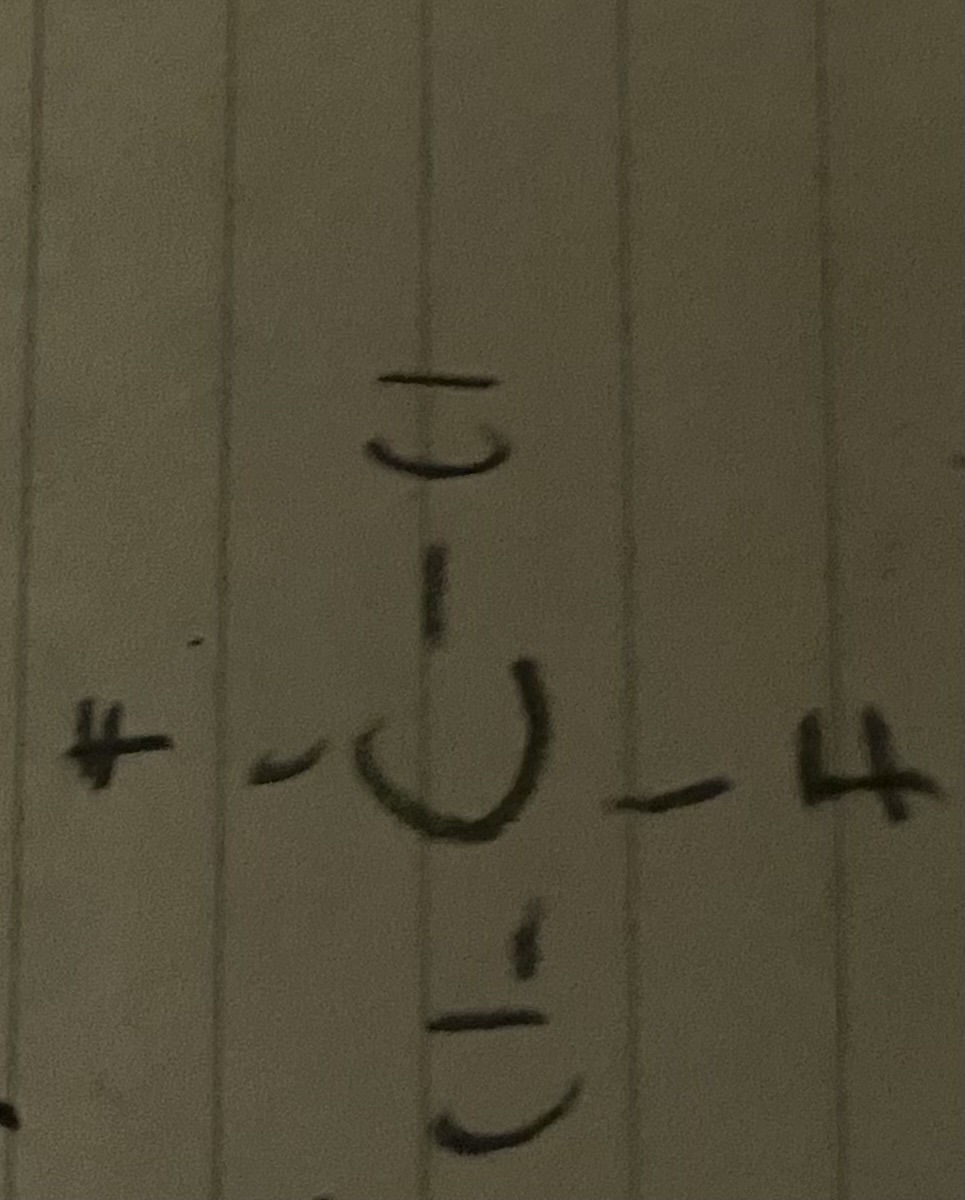
How do you name this molecule?
dichlorofluromethane
What happens during the homolytic fission?
CFC reacts with UV, removing a Cl therefore creating a radical.
The Cl will break before the F in dichloroflurocarbon as C-F has the highest bond enthalpy.
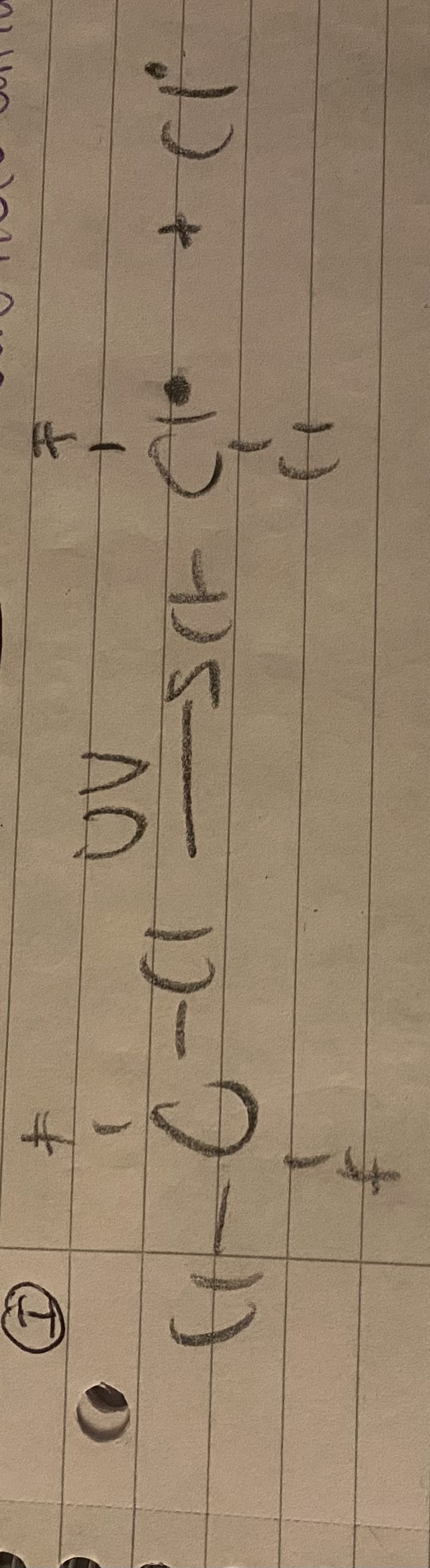
What happens during propagation?
The Cl radical reacts with O3 to form ClO radical and O2.
Then ClO radical reacts with one O molecule to form O2 and a Cl radical.
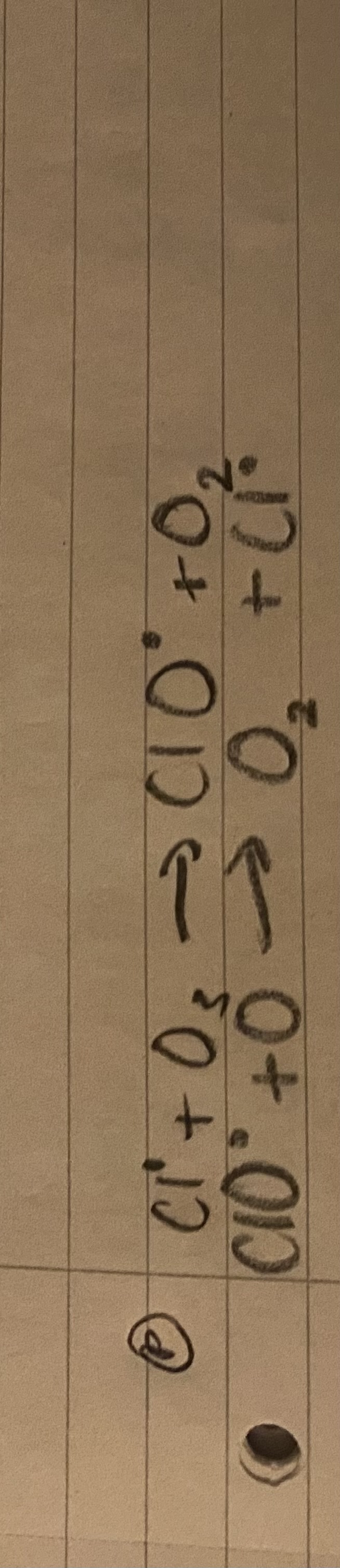
What happens in oxidation during nitrogen?
NO radical react with O3 forming NO2 radical and O2
Then the NO2 radical react with O to form NO radical and O2