PRINCIPLES OF INFECTIOUS DISEASES: ANTIMICROBIAL THERAPY AND LABORATORY MONITORING
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
ID
are caused by microorganisms including: viruses, bacteria, fungi, protozoa, parasites
transmitted by various mechanisms including: physical contact, through body fluids, consuming contaminated food or water, touching contaminated objects, airborne inhalation
Factors impacting treatment: pathogen(bugs) , antimicrobial (drug), patient (host)
Systematic approach to treating ID

Confirm the presence of infection : Fever
the average normal body temperature range taken orally is 36.7 -37 ℃ (98℉ to 98.6℉)
Normal daily temperature variation of 0.5℃ (0.9℉) and 1℃ (1.8℉) if
recovering from a febrile illness
Fever is defined as a controlled elevation of body temperature above the normal range
Aberrations of temperature reaching >38℃ (100.4℉) or <36℃ (96.8℉) are indicative of systemic inflammation
Ability to develop fever in older adults is impaired and their baseline temperature is lower, thus can have clinical implications when treating the elderly
During fever, the hypothalamus is reset at a higher temperature
Elevated body temperature, unless very high (greater than 40.5℃ [105℉]), is not harmful and may be beneficial
Check to see if antipyretics been given as they can affect patient’s temperature reading
How was temperature measured?
Oral
Rectal: 0.6℃ (1℉) higher than oral
Axillary: 0.6℃ (1℉) lower than oral
Skin: less than the oral temperature but can vary depending
on the specific measurement method
Tympanic membrane: more variable, unadjusted-mode
tympanic membrane values are 0.8℃ (1.6℉) lower than
rectal temperatures, adjusted mode similar to rectal
temperature
Forehead (temporal) scanner is usually 0.5℉ (0.3℃) to 1℉
(0.6℃) lower than an oral temperature
Confirm the presence of infection : Signs and symptoms of Infection
localized
GI: diarrhea, n/v, abdominal pain/distention
Urinary: dysuria, frequency, urgency
CNS: headache, stiff neck, photophobia, seizures
Skin/skin structure: erythema (Skin redness) , swelling, warmth, pain, purulent discharge
Respiratory: sputum production, cough, sore throat, otalgia
Other: chills, rigors
systemic
Hypo or hyperthermia
Malaise
Tachycardia
Tachypnea
Hypotension
Hypoxemia, acidosis/alkalosis
Mental status changes
Weakness
Confirm the presence of infection : Diagnostic Information Confirming Infection
imaging and scans
x rays
CT scan
MRI
others
examples
Chest X-ray or CT: consolidation, infiltrate, effusion, cavitary
nodules
Bone X-ray or MRI: bony destruction or periosteal elevation
ECHO: vegetations
Head CT/MRI: rin- enhancing lesions
Abdominal ultrasound or CT: perforation, abscess
Confirm the presence of infection : Labs : WBC
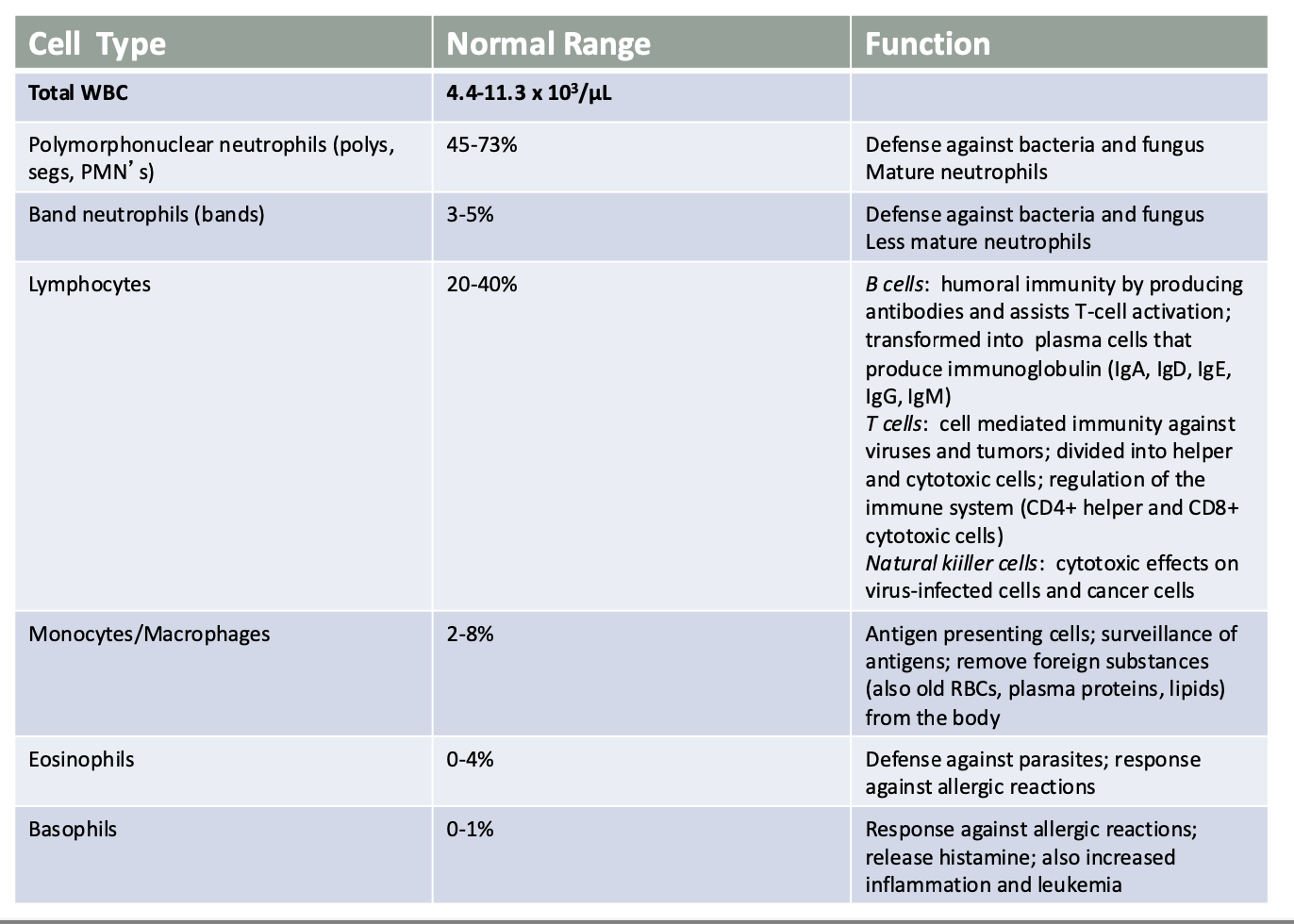
Confirm the presence of infection :Use of Other Laboratory Tests
Acute phase reactants
Elevated in inflammatory state; not specific for infection
Include: Erythrocyte sedimentation rate (ESR); C-reactive protein (CRP): shorter half-life
Large elevations in ESR and CRP are associated with infections such as endocarditis, osteomyelitis, and
pyelonephritis
ESR can be especially useful in tracking the improvement and resolution of chronic infections such as osteomyelitis, as it has demonstrable value in determining the efficacy of therapy
Procalcitonin (PCT)
Peptide precursor of calcitonin; rises in response to a
proinflammatory stimulus, especially bacterial; produced by the thyroid during calcitonin synthesis, resulting in low serum levels
During bacterial infection, PCT is produced through alternative
pathways in the spleen, kidneys, colon, brain, and lungs in response to
inflammatory cytokines
PCT levels may help determine whether to discontinue empiric
antibiotics in possibly infected patients as well as to determine
when antibiotics can be discontinued in patients recovering from infections (pneumonia)
ATS/IDSA Guidelines recommend that empiric antibiotic therapy
should be initiated in adults with clinically suspected/radiographically
confirmed CAP regardless of initial serum procalcitonin level
PCT level < 0.25 ng/mL associated with low risk of infection and can
help justify the discontinuation of antibiotics, while while levels >0.5
ng/mL may indicate antibiotics should be continued
2.Identification of the pathogen: Approach to Identifying Organism Causing Infection:
Where We Are Going
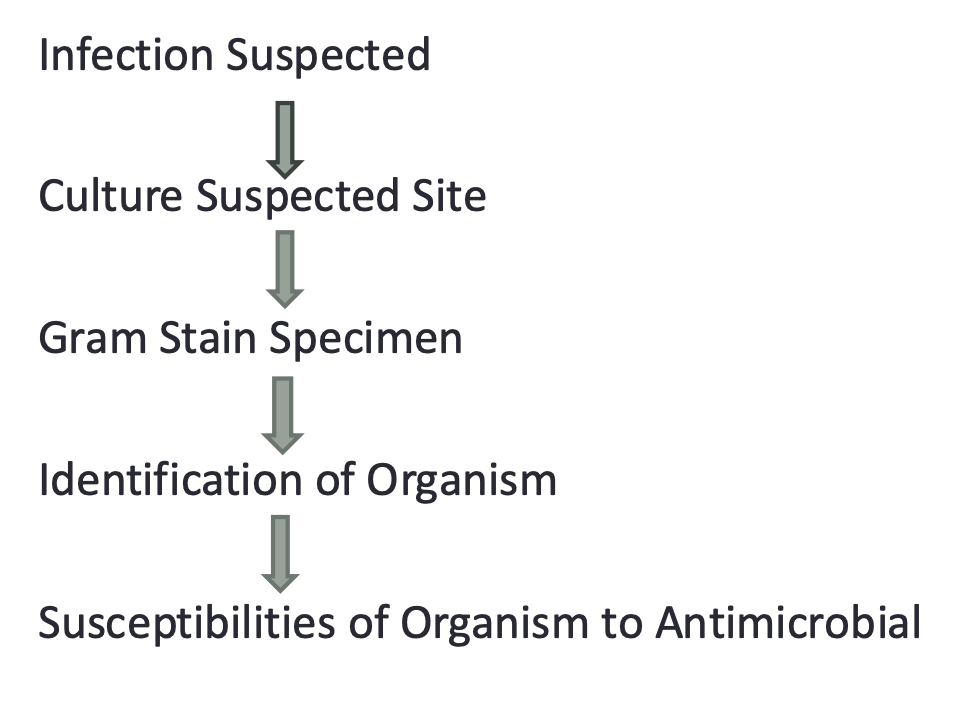
2.Identification of the pathogen: Use of the Laboratory Tests
Rapid Bacterial Detection
Helps in determining empiric antimicrobial therapy
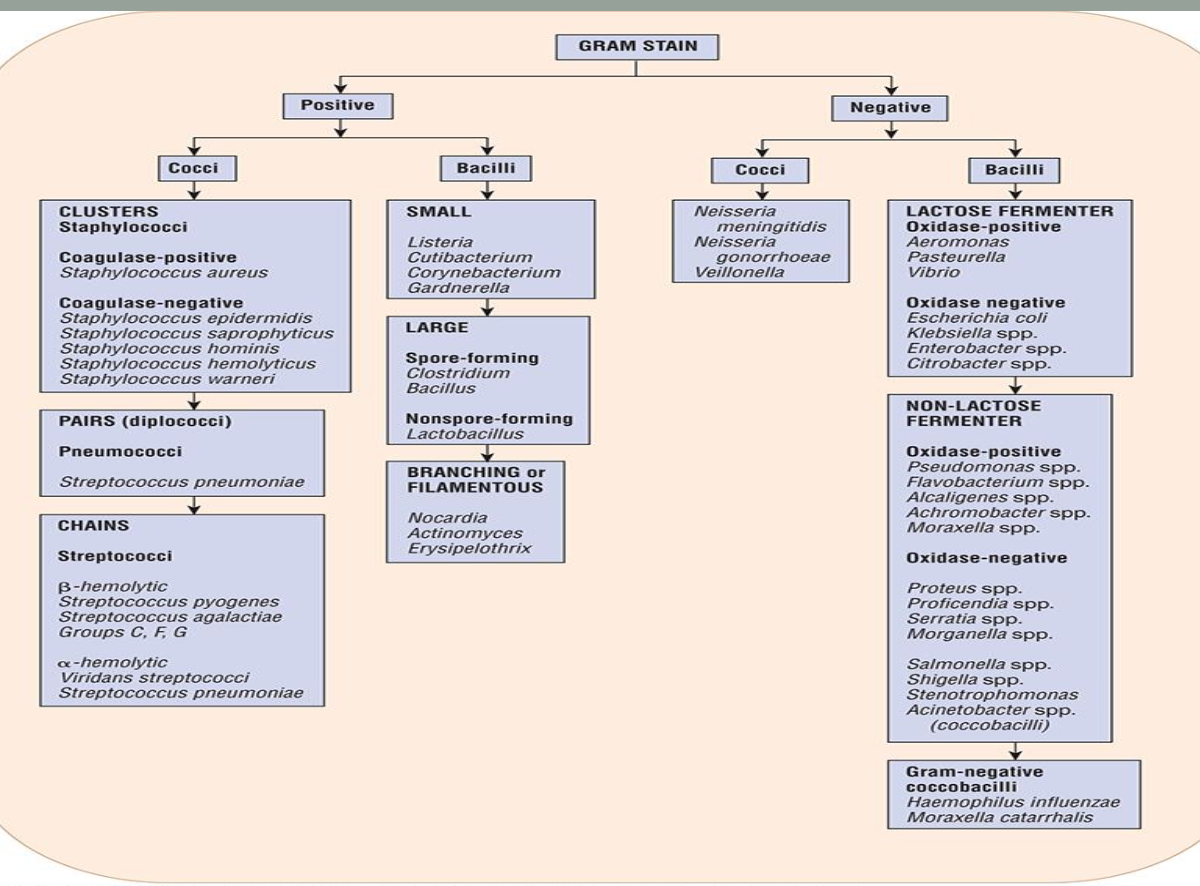
Gram Staining
Color: positive (purple), negative (pink), variable
Morphology (shape): spherical, rod, oval, spiral
Aggregation/Colony Clustering: pairs, clusters, chains
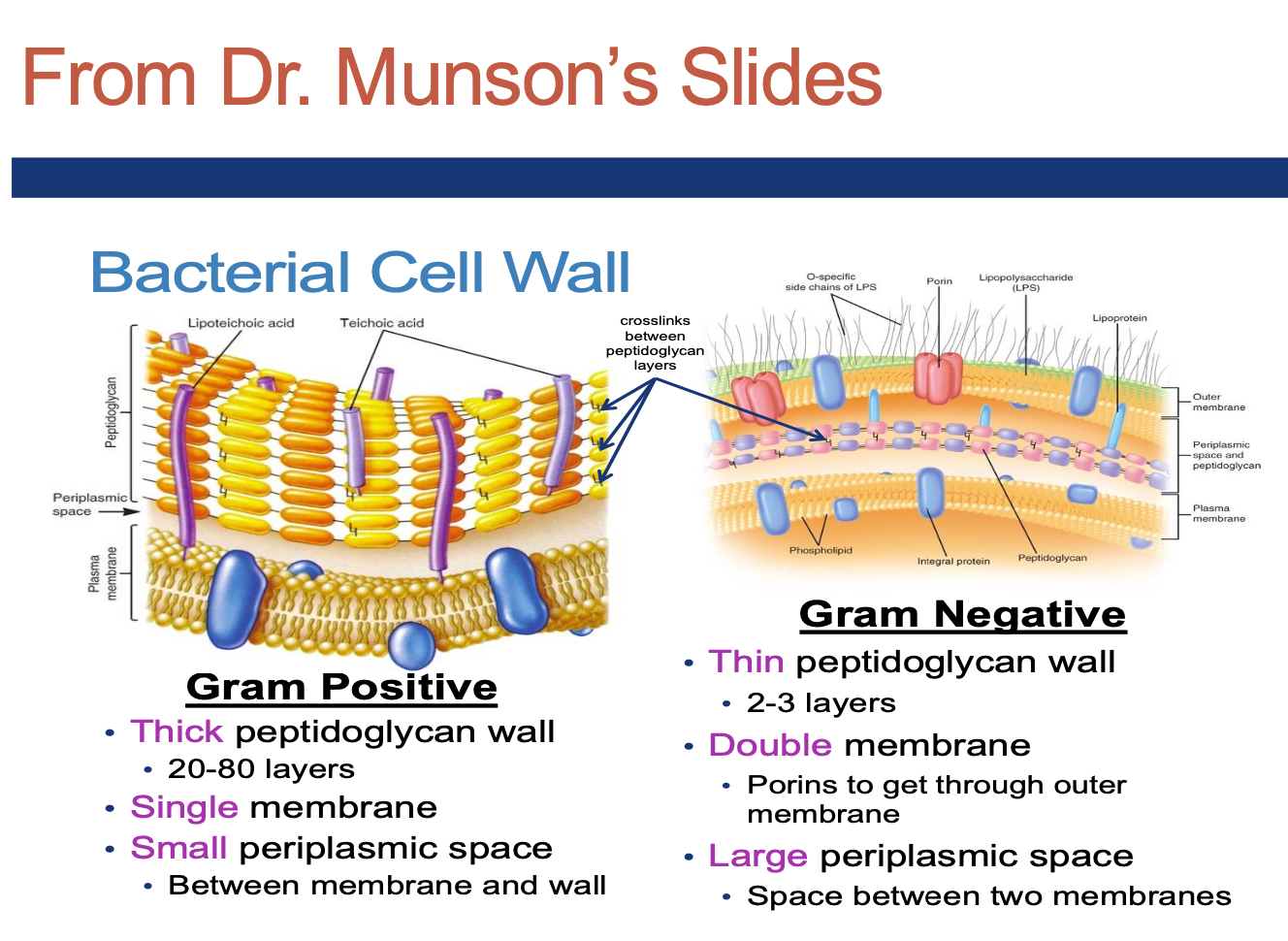
Review Dr. Munson’s slides on this and learn the basic classification of bacteria
Why do Gram-positive vs. Gram-negative bacteria stain the colors
that they do? from dr munsons
Other Bacterial Detection
Biochemistry
Rapid catalase test: catalase enzyme neutralizes the bactericidal effects of hydrogen peroxide and protects the bacteria (anaerobes generally lack the catalase enzyme)
Helps differentiate Staphylococci from Streptococci
Coagulase test: coagulase is an enzyme produced by Staphylococcus aureus that converts (soluble) fibrinogen in plasma to (insoluble) fibrin
Helps differentiate species of Staphylococci
Staphylococcus aureus (coagulase positive) which is more virulent vs.Staphylococcus epidermidis (coagulase negative) which is often a contaminant
Fermentation: ability of bacteria to metabolize sugars (glucose/lactose) to use for energy
Glucose/Lactose Fermenters: Enterobacteriaceae (E. Coli, Klebsiella, Serratia, Enterobacter)
Nonfermenting: Pseudomonas, Acinetobacter, Stenotrophonmonas, Burholderia
Oxidase test: used to identify bacteria that produce cytochrome c oxidase, an enzyme of the bacterial electron transport chain (and can
use oxygen for energy production by converting O2 to H2O2 or H2O with an electron transfer chain)
Oxidase positive: Pseudomonas, Pasteurella , Moraxella, others
Oxidase negative: Enterobacteriaceae, others
Appearance on agar
Hemolysis: Some bacteria produce exoenzymes (called hemolysins) that lyse red blood cells and degrade hemoglobin (Beta-hemolysin breaks down the red blood cells and hemoglobin completely (clear zone); Alpha-hemolysin partially breaks down
the red blood cells (green color due to biliverdin)
Helps differentiate species of Streptococci
⍺-hemolytic: Streptococcus pneumoniae, viridans Streptococci
Β-hemolytic: Group A Streptococcus (pyogenes), Streptococcus
agalactiae, Streptococcus Groups C, F, G
Nonhemolytic: Enterococci
2.Identification of the pathogen : Use of Laboratory Tests
Slow Bacterial Detection
Fastidious Organisms: grow slowly (few days to weeks) and often require special media and nutrients
Examples: Mycobacterium, Haemophilus, Campylobacter, Helicobacter, Neisseria, Bartonella, Listeria, Legionella, Chlamydia, HACEK organisms (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, Kingella)
Other staining
Ziehl–Neelsen stain
Detect acid-fast bacilli, which is used for the identification of mycobacteria species by making them appear bright red against a blue or green background
India ink, potassium hydroxide (KOH), Grocott’s Methenamine Silver (GMS), others
Detect fungi
Gram Stain
2.Identification of the pathogen :Use of Laboratory Tests: Rapid Diagnostic Testing (RDT)
Traditional diagnostic testing for identification/susceptibility can take at least 48-72h, whereas RDT’s can significantly reduce time by at least 24h
Use techniques such as polymerase chain reactions (PCR) or matrix-associated laser desorption-ionization time-of-flight (MALDI-TOF) mass spectrometry that don’t relay on culturing
Common types include antigen, antibody, and nucleic acid amplification tests
Have been shown to:
Decrease: time to effective and optimal therapy, antibiotic utilization, length of stay, mortality, costs
Increase: clinical cure rates, likelihood of ID consult
Target microorganisms associated with increased morbidity and mortality including: Candida, Clostridioides difficile, MRSA nasal swabs, viruses (HIV,influenza, COVID), malaria, Group A Streptococcus (Rapid Strep Test), gram negative and gram positive bacteremias, meningitis, others
2.Identification of the pathogen :Use of Laboratory Tests: Culture and Susceptibility
(24-72 hours)
Most definitive method for diagnosis and treatment of an
infection
Sites tested determined by suspected site of infection (urine, blood, CSF, sputum, etc.)
Provides initial identification of organism by:
Gram stain
Growth on selective media
Presence or absence of enzymes
Chemical characteristics
Definitive identification of organism follows
Susceptibility (sensitivity) of organism to antimicrobial agent
2.Identification of the pathogen :Use of Laboratory Tests: MIC and MBC
Minimum Inhibitory Concentration (MIC): Lowest concentration
of drug that will inhibit visible growth
Organism is isolated and drug added in vitro to determine
Minimum Bactericidal Concentration (MBC): Lowest
concentration of drug that kills the bacteria or results in 99.9% reduction of the initial inoculum
Organism is isolated and drug added in vitro to determine
Subculture of tubes showing inhibition of growth
More difficult to perform and not usually done in clinical practice
2.Identification of the pathogen :Use of Laboratory Tests
Sensitivity Testing Methods
Disk Diffusion (Kirby-Bauer)
Tube-dilution
Automated
Vitek System
Microscan Walkaway System
BD Phoenix Automated Microbiology System
Epsilometer test (E test)
Interpretative Guidelines (based on breakpoints set by Clinical and Laboratory Standards Institute [CLSI])
Sensitive (susceptible)
Intermediate (moderately susceptible)
Resistant
![<ul><li><p><strong> Sensitivity Testing Methods</strong></p><ul><li><p>Disk Diffusion (Kirby-Bauer)</p></li><li><p>Tube-dilution</p></li><li><p>Automated</p><ul><li><p> Vitek System</p></li><li><p>Microscan Walkaway System</p></li><li><p>BD Phoenix Automated Microbiology System</p></li></ul></li><li><p>Epsilometer test (E test)</p></li></ul></li><li><p><strong>Interpretative Guidelines (based on breakpoints set by Clinical and Laboratory Standards Institute [CLSI])</strong></p><ul><li><p>Sensitive (susceptible)</p></li><li><p> Intermediate (moderately susceptible)</p></li><li><p>Resistant</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f8e1c614-606c-4c4f-98c7-651fe2dd6a2a.png)
Use of Laboratory Tests
Bactericidal:
Antibiotic kills the bacteria without needing help from the patient’s immune system (preferred for endocarditis, meningitis, osteomyelitis, neutropenic patients)
Bacteriostatic:
Antibiotic inhibits growth of the bacteria without killing it, but is usually successful in treating infection because the immune system of the patient can kill the bacteria
Microdilution MIC Testing: commonly used in micro labs
and in automated systems
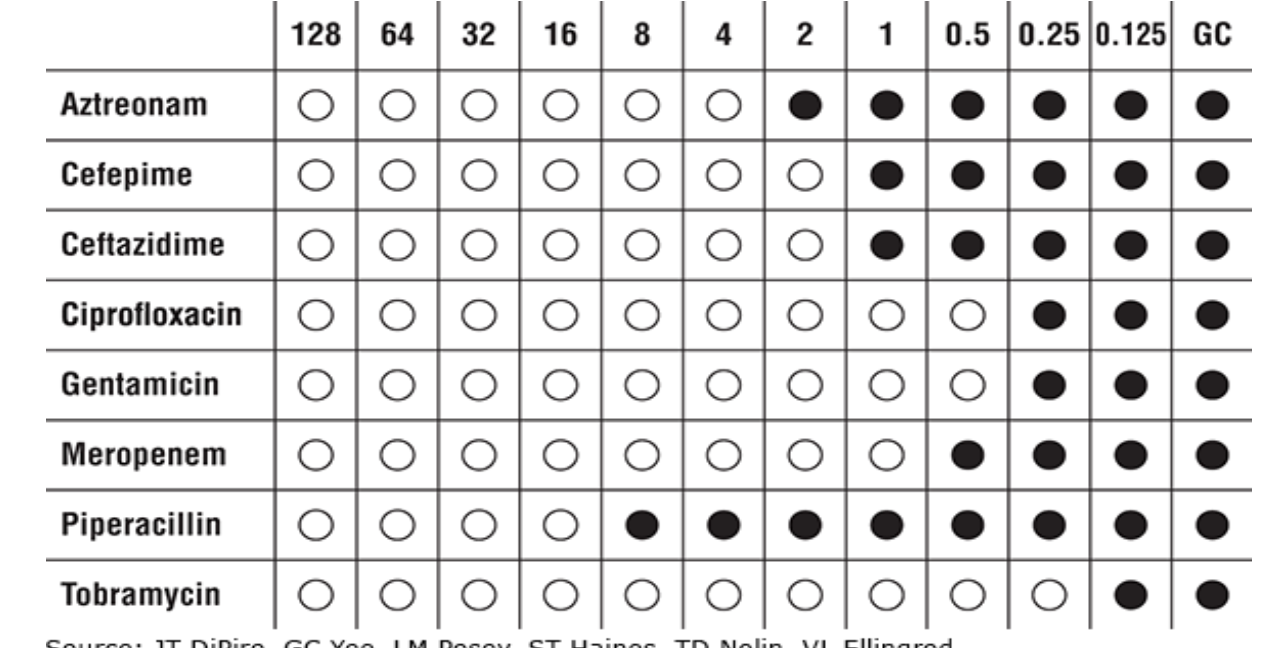
Sensitivity Testing Methods: Epsilometer Test (Etest)
Plastic strip with fixed concentration gradient of an antibiotic placed on an agar plate streaked with bacteria
Bottom of ellipse crossing strip is the MIC
Employed for Streptococcus pneumoniae, some gram negatives
Antibiotic Susceptibility : MIC Breakpoints
MIC Breakpoint: concentration at which antibiotic and
bacteria is considered susceptible, intermediate, resistant
Standards published by Clinical and Laboratory Standards Institute (CLSI) and set by FDA upon drug approval process
Breakpoints established by achievable serum concentrations of the antibiotic after normal dosing, inherent susceptibility of the organism to the antibiotic, site of infection, and results of efficacy trial
Breakpoints vary for an antibiotic based on the particular bacteria and site of the infection
Done to help predict the probable response of an infection to an antibiotic
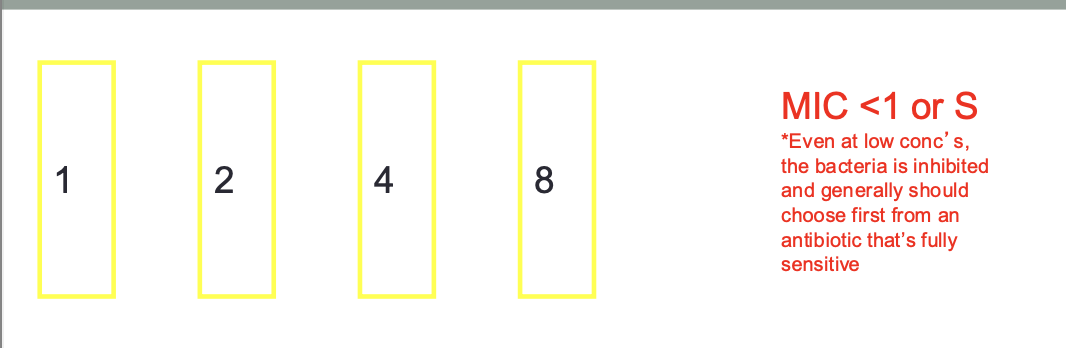
MIC Breakpoints. : Susceptible
bacteria tested has a low MIC (very sensitive) will most likely be eradicated since concentrations (represented by the MIC) are easily achievable by standard doses employed
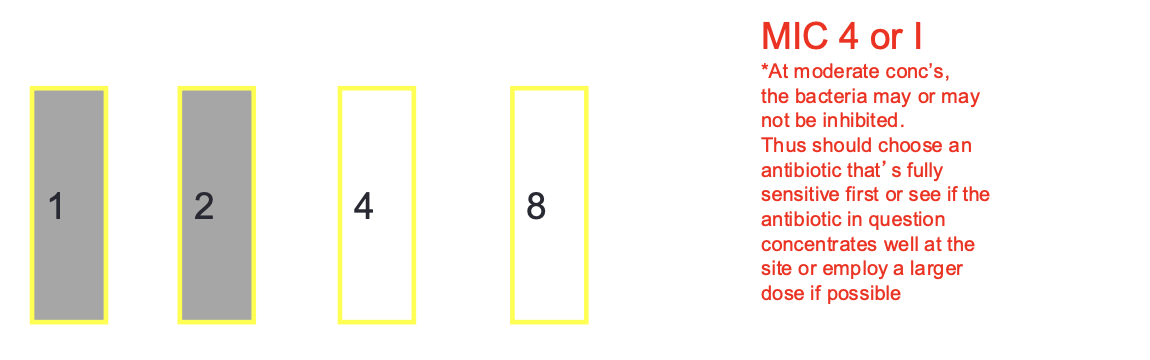
MIC Breakpoints: Intermediate
bacteria tested has a higher MIC and thussuccessful treatment may or may not occur or might possibly employ a higher dose
MIC Breakpoints:Resistant
bacteria tested has an extremely high MIC (not sensitive) that exceeds the achievable serum concentration of the antibiotic even if high doses are
used, and the strain would not be inhibited and poor patient response would be expected
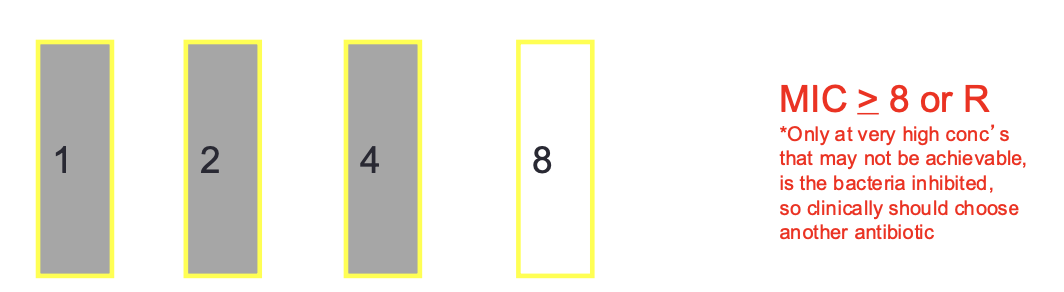
Antibiotic Susceptibility Breakpoints
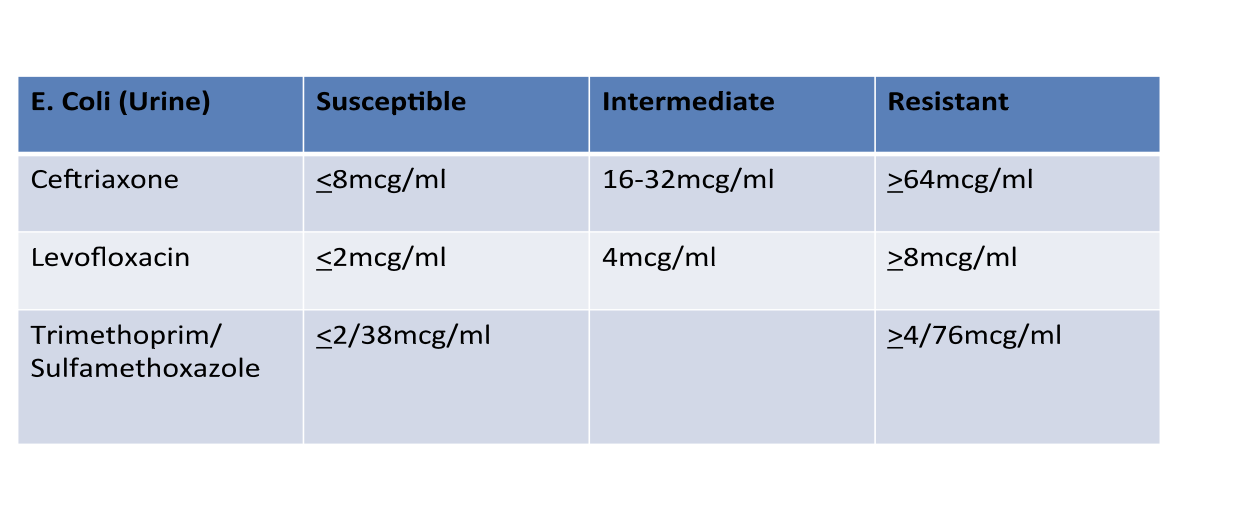
Antibiotic Susceptibility Testing
Clinical Pearls
Just because an antibiotic has the lowest MIC for an organism does not mean it’s the best antibiotic to use because different antibiotics achieve different concentrations in the body depending on the site of infection: MIC’s are specific to the microorganism and antimicrobial
Remember that the in vitro efficacy of the susceptibility tests may not translate into in vivo efficacy in the patient due to multiple factors
Other factors need to be considered—make sure always make the clinical correlation
Site of infection
Severity of infection
Pharmacokinetics
Concomitant disease states, allergies, etc.
Spectrum of activity (narrow preferred)
Efficacy from clinical trials/drug of choice
Cost
Sensitivity Testing: Other Microorganisms
Historically, mycobacteria, fungal, and viral sensitivity testing
has not been well defined
More recent advances have improved this
Radiometric techniques (BACTEC TB460 with results within 1 week, BACTEC Mycobacteria Growth Indicator Tube [MGIT 960]) for M. tuberculosis and other slow growing mycobacteria
Fungal sensitivities much more reliable and reproducible and now CLSI guidelines for
Hospital Antibiograms
Cumulative report of the antimicrobial susceptibility profiles of
the organism isolated with a hospital and surrounding
community
Reports the percent of isolated organisms that were susceptible
to different antibiotics over a certain time frame (yearly, twice
yearly, etc.)
Useful for selecting the most appropriate empiric antibiotic
May be done in different areas of hospital as well
use
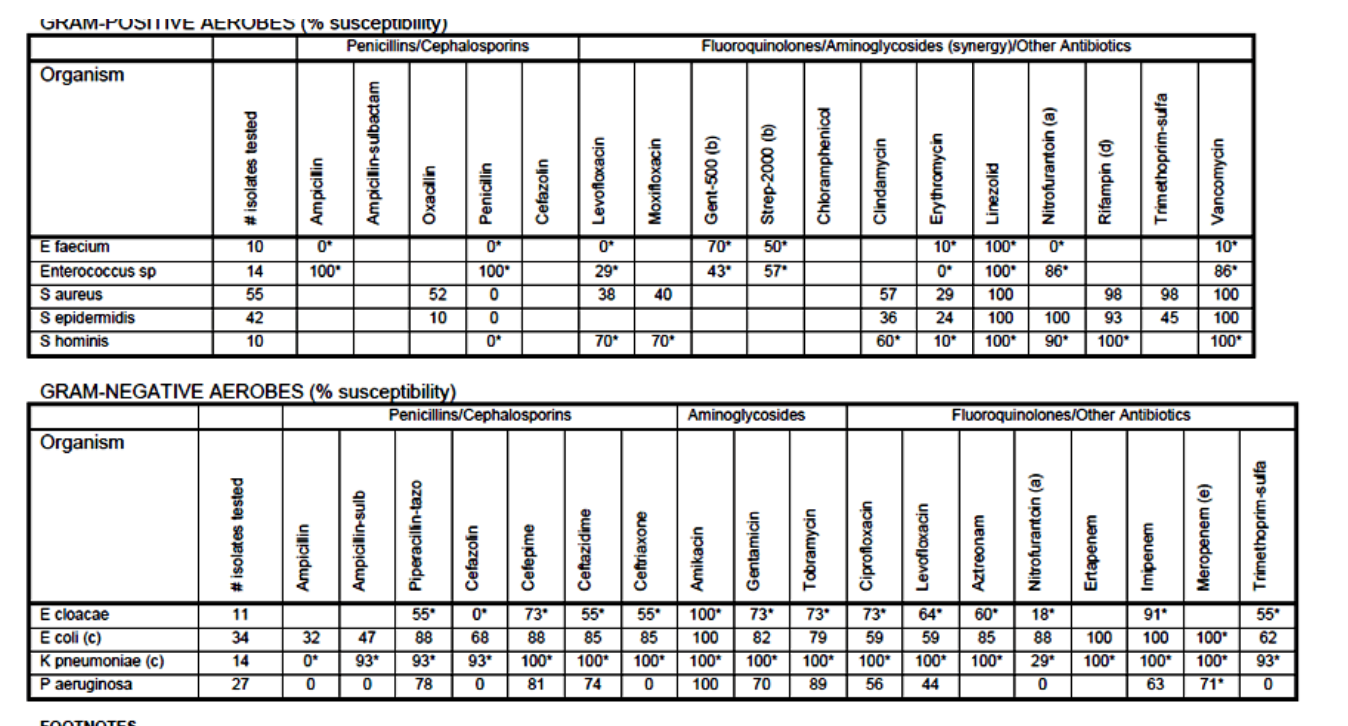
use of laboratory tests : Serum Antibiotic Assay
Can be done with any drug
Various methods to perform in lab:
Fluorescence-Polarization Immunoassay (TDx system)— most common
Radioimmunoassay (RIA)
High-Pressure Liquid Chromatography (HPLC)
Most useful for drugs with narrow therapeutic index to
maximize efficacy and minimize toxicity
Examples: aminoglycosides, vancomycin
Types of levels: peak, trough, random levels
Pharmacokinetic/Pharmacodynamic
Parameters and Dose Optimization
Peak/MIC ratio (concentration-dependent)
Need to optimize dose to produce higher drug concentrations
Aminoglycosides, quinolones, daptomycin
Goal: high peak (increased bacterial killing), low trough (decreased toxicity)
Dosing: large dose, long dosing interval
AUC/MIC ratio (exposure-dependent)
Need to optimize dose and exposure to unbound drug concentrations
Vancomycin (AUC/MIC>400-600), macrolides, tetracyclines, polymyxins
Goal: exposure over time
Dosing: variable
Time above MIC (time-dependent)
Optimize duration unbound concentration at or above the MIC
Beta lactams (penicillins, cephalosporins, carbapenems)
Goal is to have drug levels above the MIC for most of the dosing interval
Dosing: shorter dosing interval, extended or continued infusions
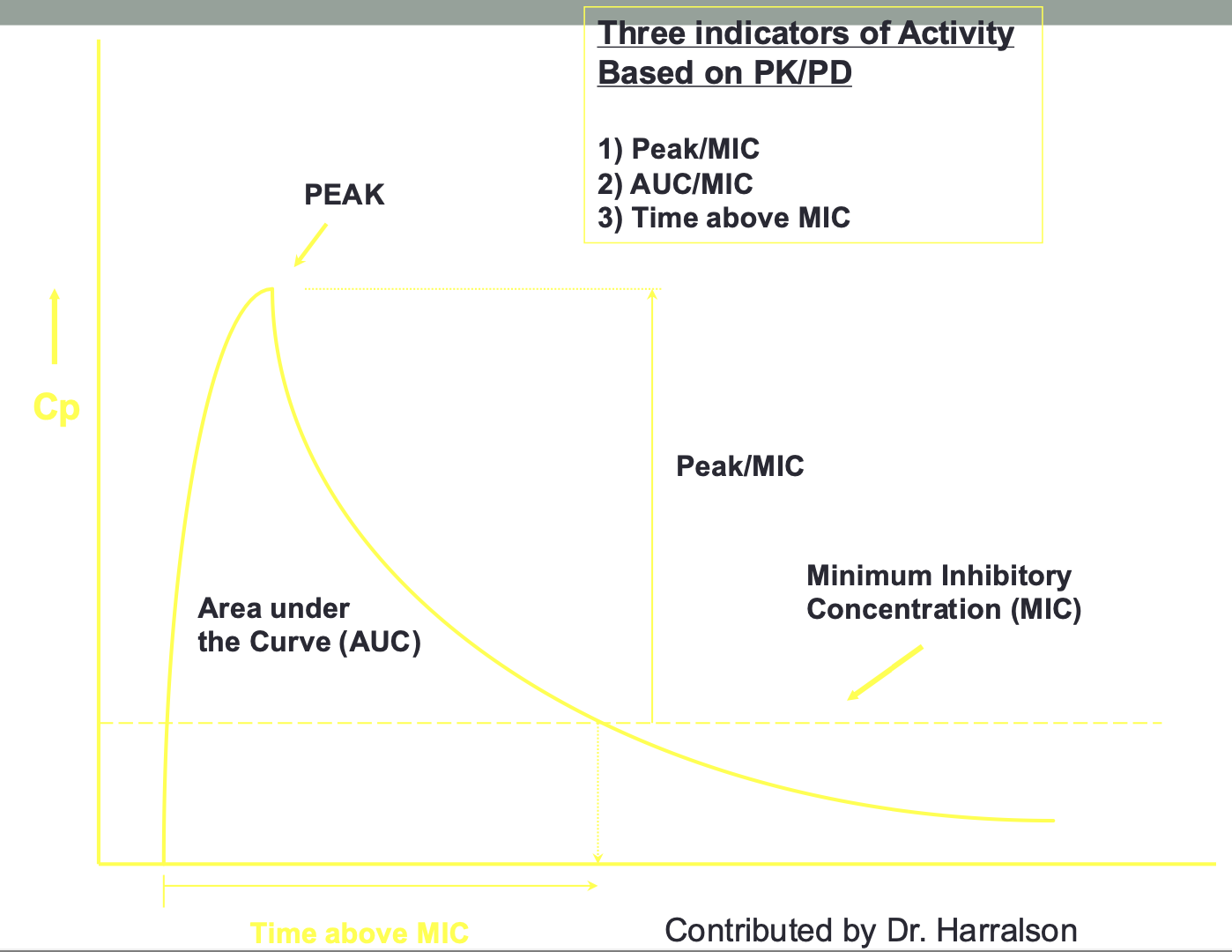
use of laboratory tests: Postantibiotic Effect (PAE)
Continued suppression of bacterial growth after the antibiotic serum levels fall below the MIC
Can be tested in lab by exposing bacteria to the antibiotic, and then removing/inactivating the antibiotic, and then time recorded on how long takes bacteria to regrow; quantified as time takes for organism to demonstrate 10-fold increase in viable cells (not usually tested in clinical practice)
Clinically applied for extended interval aminoglycosides and some other antibiotics to allow for less frequent administration
Selection of presumptive therapy:Types of Infection
Community-Acquired Infection
Infection originating in the outpatient or community setting or is present on admission
No recent hospitalization or invasive medical procedure
Hospital-acquired (or nosocomial) Infection
Infection occurs 48 hours or more after admission and did not appear to be incubating at the time of admission
Selection of presumptive therapy:Describing ID States:
Colonization
microorganisms do not invade the host and are part of the normal flora of the site without host inflammatory responses
Selection of presumptive therapy:Describing ID States: Contamination
the presence of microorganisms typically acquired during acquisition or processing of specimens without host inflammatory response
Selection of presumptive therapy:Describing ID States: Infection
microorganisms invade the host and with host inflammatory response (signs/symptoms of infectious process)
Sterile anatomical sites:
CSF, blood, lungs, urinary tract, biliary
tract
Microorganisms if found usually pathogenic, although could be colonization or contamination
Clinical correlation is important
Non-sterile anatomical sites:
sputum, pus, skin, GI tract, vagina
Microorganisms expected to grow
Clinical correlation still important
Rational
Antibiotic
Selection
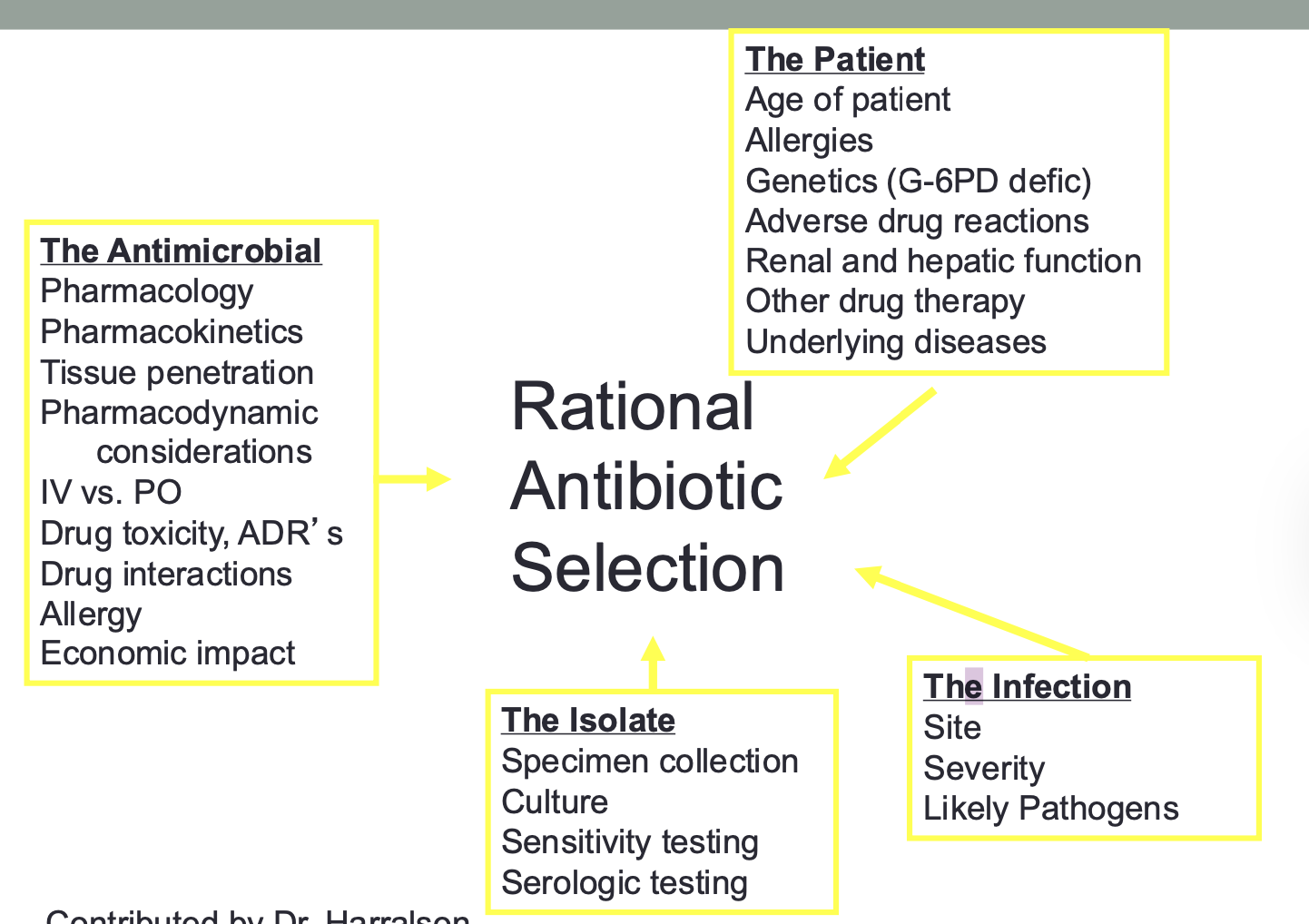
Types of ID Treatment: Empiric
Initial broad antimicrobial spectrum before identification of the organisms directed against the organisms know to cause the infection in question based on patient’s presentation
Types of ID Treatment: Definitive:
Antimicrobials selected based on clear identification of the organism(s) and proven sensitivity of the organism(s)
Types of ID Treatment: Prophylactic:
Antimicrobial directed against a single pathogen or multiple pathogens to prevent an infection from occurring; is usually short-term (before surgery, dental procedures) but can be long-term (AIDS)
Combination Antimicrobial Therapy
Advantages
Broadening the spectrum of coverage : Necessary in mixed infections where multiple organisms expected (ex. intraabdominal infections)
Synergism: Certain infections such as Enterococcus endocarditis
(penicillin or ampicillin + gentamicin or streptomycin)
Prevent development of resistance
Used in tuberculosis
Other infections: data not as convincing
Antibiotic Combinations :Antimicrobial Combination Effect Test
Done by microtiter fractional inhibitory concentration (“checkerboard” method) or timed-kill curves
Not routinely done in clinical practice
Categories
Synergy: Greater activity than the sum of activity of either agent alone
Antagonism: Activity that is worse than either agent alone
Additive/Indifferent: Activity that is neither synergistic or antagonistic
Enterococcus—susceptibility to high concentrations of aminoglycosides (gentamicin 500 mg/mL) is evaluated in the lab because it correlates closely with synergy when combined with beta lactam antibiotics (endocarditis treatment)
Combination Antimicrobial Therapy
Disadvantages
• Increased costs
• Increased risk of toxicity
• Superinfection with resistant organisms
• Antagonism
Monitor therapeutic response: ID Monitoring
After antimicrobial therapy has been started, monitoring of therapeutic response important
Culture and sensitivity reports should be reviewed and therapy adjusted accordingly
Antimicrobials with narrowest spectrum of activity against identified pathogens recommended (streamlining therapy)
If anaerobes are suspected even if not identified, anaerobic therapy should be continued
Patient monitoring should include many of the same parameters used to diagnose the infection
WBC and temperature should start to normalize
Physical complaints from the patient also should diminish (decreased pain, shortness of breath, cough, or sputum production)
Appetite should improve
Radiologic improvement can lag behind clinical improvement
Monitor serum (or other fluid) levels of antimicrobials to ensure outcome, preventing toxicity, or both (aminoglycosides, vancomycin, others)
Monitor for changes in Vd, clearance
Streamline route of administration (IV to PO switch) if possible
Overall clinical improvement, lack of fever for 8 to 24 hours, decreased WBC, functioning GI tract
Drugs with good BA
Antimicrobial failure
If fail to respond over 2 to 3 days, then reevaluate
Disease may not be infectious or is nonbacterial in origin or there is an undetected pathogen
Other factors: drug selection, the host, microorganism (resistance), laboratory error in identification or susceptibility testing or both (presence of inoculum effect or resistant subpopulations)
Antimicrobial Stewardship Programs (ASP’s) designed to promote effective use of antimicrobials
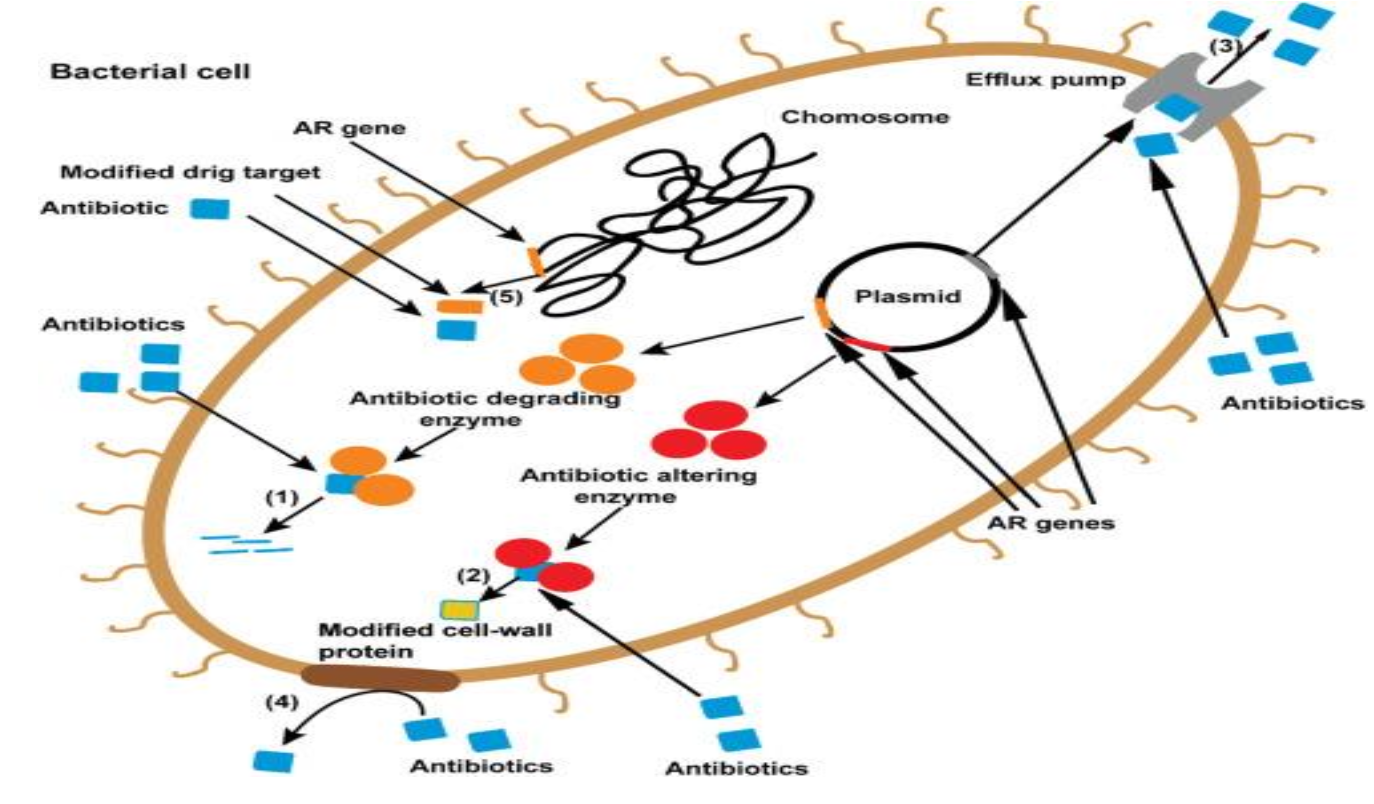
Antibiotic Resistance
Ability of bacteria to grow in the presence of an antibiotic that
normally limits its growth or kills it
Significant problem resulting in antibiotic failure and significant
morbidity and mortality
Mechanisms of resistance:
Intrinsic: natural to the bacteria (ex. vancomycin resistance to gram negative bacteria because is too large to penetrate cell wall)
Selection Pressure: antibiotics eliminate susceptible bacteria leaving behind more resistance bacterial strains to grow
Acquired: bacterial DNA containing resistant genes can be transferred between different species or obtained from dead environmental bacterial fragments
Enzyme Inactivation: bacteria produce enzymes that inactivate the antibiotic (examples include beta lactamases, extended-spectrum beta- lactamases (ESBLs), carbapenem-resistant Enterobacteriaceae (CRE)
Common Resistant Bacteria
Klebsiella pnuemoniae (ESBL, CRE)
Escherichia coli (ESBL, CRE)
Acinetobacter baumannii
Enterococcus faecalis, Enterococcus faecium (VRE)
Staphylococcus aureus (MRSA)
Pseudomonas aeruginosa
Pneumonic: Kill Each and Every Strong Pathogen
Antibiotic Resistance Mechanisms