qs weakness bank chem
1/24
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
describe movement particles in a gas (1)
particles in a gas are constantly moving with random motion in straight lines
describe what happens to the particles ina slid when it is heated to the point of melting (4)
particles gain energy
and vibrate more
This weakens the forces that hold the solid together and makes the solid expand
At the point of melting, many of the particles have enough energy to break free from their positions
In the experiment shown in the diagram, a gas jar full of brown bromine gas is separated from a gas jar full of air by a glass plate.
The glass plate is then removed.
Describe and explain the appearance of the gas jars after an hour. (2)
Both jars will be the same paler brown colour
because the random motion of the bromine and air particles means that they will eventually be equally mixed throughout both jars
define solution (2)
A mixture of a solute and a solvent
that does not separate out /1 mark).
The student keeps adding sodium chloride to the beaker and stirring the solution.
Eventually, the sodium chloride starts to settle at the bottom of the beaker. Explain why. (1)
The solution had become saturated, so no more sodium chloride can dissolve
relative mass e- (1)
negligible or 0.0005
name group atoms held together by cov bonds (1)
a molecule
state what is meant by the term isotope (2)
Isotopes are different atomic forms of the same element, which have the same number of protons
but a different number of neutrons
Briefly describe the difference between an element and a compound (2)
elements consist of one type of atom only
compounds are made of two or more different elements which are chemically bonded together
Copper can be made extremely pure. The melting points of two samples of copper were measured. Sample A had a melting point of 1085 °C and sample B melted over the range 900 - 940 °C.
Suggest which of the samples, A or B, was the most pure. Explain your answer. (2)
Sample A
The purer the substance, the smaller the range of the melting point
pure substances have sharp melting points, whereas impure substances melt over a range of temperatures
The photograph shows the NASA Space Shuttle soon after being launched.
The large central tank contains liquid oxygen and liquid hydrogen.
In the shuttle's thrusters, oxygen reacts with hydrogen to produce water vapour.
State whether each of the substances below is an element, compound or mixture.
Explain your answer in each case. (4)
Water vapour is a compound
because it is made up of two different elements which are chemically bonded together
Liquid oxygen is an element
because it consists of one type of atom only
Magnesium is found in group 2 and period 3 of the periodic table.
Explain how you could use this information to deduce the electronic structure of magnesium. (3)
The group number tells you how many electrons are in the outer shell, so magnesium has 2 outer shell electrons
The period number tells you how many electron shells the atom has in total, so magnesium has three shells
All the shells apart from the outer shell will be filled (the first holds 2 electrons and the second holds 8)
Some light bulbs contain a thin metal filament. If these bulbs were filled with air, oxygen would react with the filament causing it to burn away. To avoid this, the light bulbs are filled with argon.
Explain why argon is suitable for this use (2)
Argon won't easily give up or gain electrons, making it inert
This means that it won't react with the metal filament in the light bulb
Potassium chloride (KCI) is an ionic compound containing potassium ions bonded to chloride ions.
Describe the bonding between the potassium ions and chloride ions in potassium chloride. (2)
Sodium atoms can react to form sodium ions.
a) Sulfur and chlorine both react with sodium to form ionic compounds.
Explain how you can use the positions of sulfur and chlorine in the periodic table to predict the number of electrons they will gain when they form ions. (2)
b) Sodium nitrate (NaNO,) is an ionic compound containing sodium ions bonded with nitrate ions.
Explain why nitrate ions cannot form ionic compounds with bromide ions. (2)
a)
The number of electrons gained is equal to
8 - the group number
Sulfur is in Group 6, so 8 - 6 = 2 electrons gained. Chlorine is in Group 7, so 8 - 7 = 1 electron gained
b)
Both nitrate and bromide ions are negatively charged
Ionic compounds can only be formed between negative and positive ions
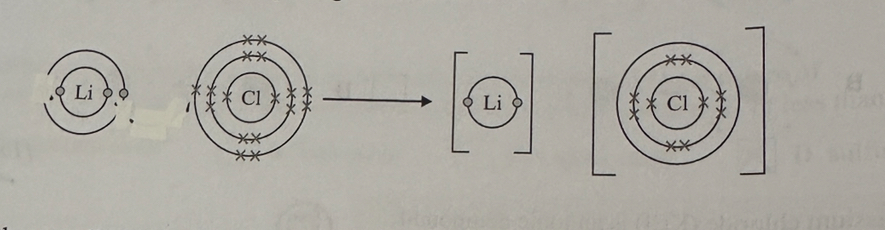
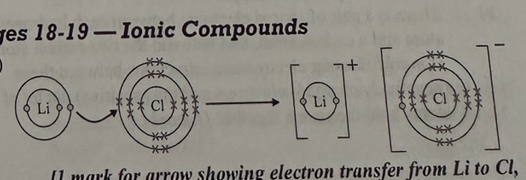
The diagram below shows the formation of the ionic compound lithium chloride from its elements, but it is incomplete.
a) Complete the diagram below by drawing an arrow to show the transfer of the electron, adding the charges of the ions and completing the chloride ion to show the electrons in its outer shell. (3)
b) State the overall charge of lithium chloride. (1)
a)
1mark for arrow showing electron transfer from Li to C
1 mark for adding seven crosses and one dot to outer shell of the chloride ion
1 mark for correct charges on both ions.
b)
neutral/no overall charge
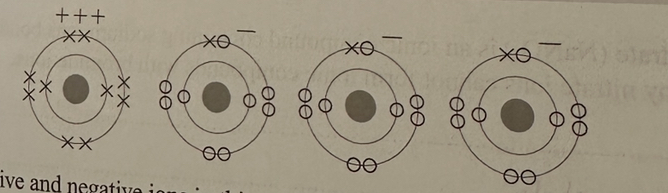
The diagram below shows the ions in an ionic compound. All of the electrons are shown.
Identify the positive and negative ions in this compound. (2)
positive: AI3+
negative: F-
From the dots and the crosses, you can see how many electrons each ion has gained or lost. You can use the periodic table to work out what atom it was to start with.
A student reacts magnesium nitrate with potassium hydroxide.
The products of the reaction are magnesium hydroxide and potassium nitrate.
Write the chemical formulae of magnesium hydroxide and potassium nitrate. (2)
magnesium hydroxide: Mg(OH)
potassium nitrate: KNO
Sodium chloride is an ionic compound.
a) Describe the structure of a crystal of sodium chloride. You should state:
• What particles are present in the crystal.
• How these particles are arranged.
• What holds the particles together. (4)
b) Explain why sodium chloride has a high melting point. (2)
a)
Sodium chloride contains positive sodium ions (Na*)
and negative chloride ions (CI)
that are arranged in a giant ionic lattice
The oppositely charged ions are held together by a strong electrostatic attraction
b)
To melt sodium chloride, you have to overcome the very strong electrostatic forces/ionic bonds between the ions
which requires lots of energy
Silicon has the electronic structure 2.8.4.
Use this information to predict the maximum number of covalent bonds one atom of silicon can form in a simple molecule. Explain your answer. (2)
A silicon atom has four electrons in its outer shell
So a silicon atom can form four covalent bonds
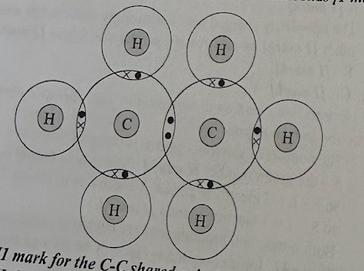
Hydrocarbon gases such as ethane (C,H.) are useful chemicals.
a) Draw a dot and cross diagram of ethane. Only show the outer electrons of each atom.
b) Explain how the carbon and hydrogen atoms are held together in a molecule of ethane. (2)
a)
1 mark for the C-C shared pair
1 mark for all of the H-C shared pairs correct
b)
There is a pair of shared electrons between each hydrogen atom and a carbon atom, and between the two carbon atoms
Strong electrostatic attractions between these (negatively charged) electrons and the (positive) nuclei of the atoms hold the atoms together
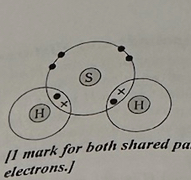
The atoms in hydrogen sulfide (HS) are bonded in a similar way to the atoms in water (H,O). (2)
Draw a dot and cross diagram of hydrogen sulfide. Only show the outer electrons of each atom. (2)
1 mark for both shared pairs
1 mark for the non-bonding electrons
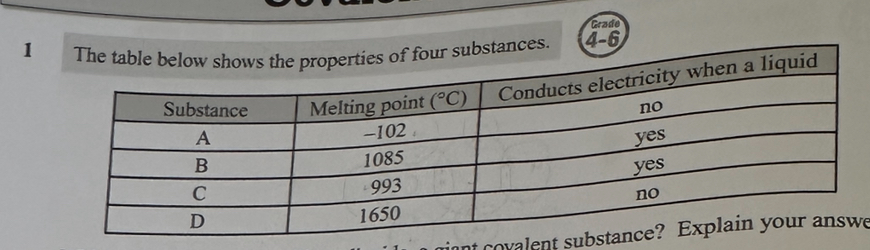
Which substance could be silicon dioxide, a giant covalent substance? Explain your answer. (2)
D
giant covalent substances have, high melting points and don’t normally conduct electricity, even when molten
Silicon carbide has a giant covalent structure and is a solid at room temperature.
Explain, in terms of its bonding and structure, why silicon carbide has a high melting point. (2)
In a giant covalent structure, all of the atoms are bonded to each other with strong covalent bonds
It takes lots of energy to break the many bonds and melt the solid
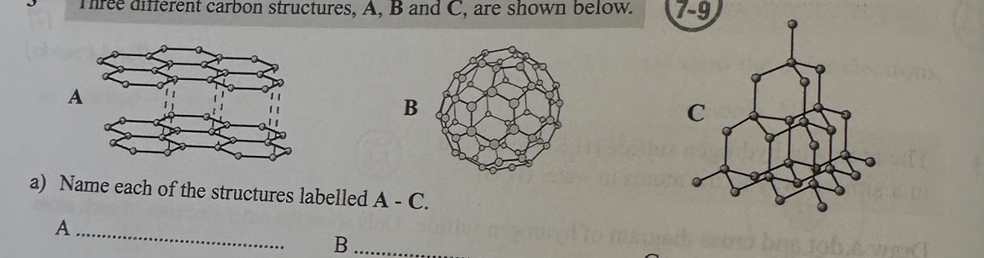
Three different carbon structures, A, B and C, are shown below.
a) Name each of the structures labelled A - C. (3)
b) Explain how the bonding in structures A and C affects their electrical conductivity and hardness. (4)
c) A and B both sublime. Explain why a sample of B would sublime at a lower temperature than A. (3)
a)
A - graphite
B — C60 fullerene
C — diamond
b)
Graphite/structure A can conduct electricity because it contains delocalised electrons that can move around
Diamond/structure C doesn't conduct electricity because it doesn't contain any charged particles that can move around
Diamond/structure C is hard because the strong covalent bonds hold the atoms in a rigid lattice structure
Graphite/structure A is soft, as the layers
of graphite are held together by weak intermolecular forces
c)
Structure B is a simple molecular substance
so only weak intermolecular forces need to be broken to separate the molecules
In order for A to sublime, strong covalent bonds need to be broken, which would require more energy