lecture 6 - introduction to tissue mechanics
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
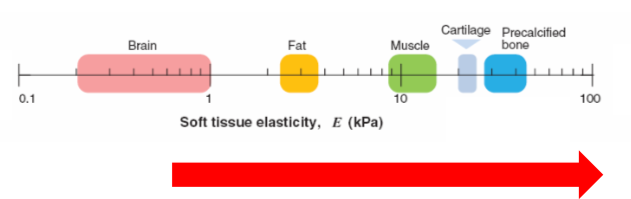
tissue microenvironments are physically diverse
measure stiffness through elasticity
calcified bone is very stiff
brain is soft
heart is stiff
bone is rigid
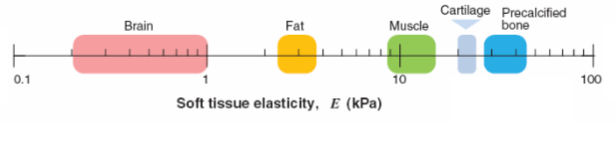
young’s modulus(E)
it is a measure of stiffness or elasticity
measures how much force it takes to deform something
the higher it is on the scale, more force is needed to deform it
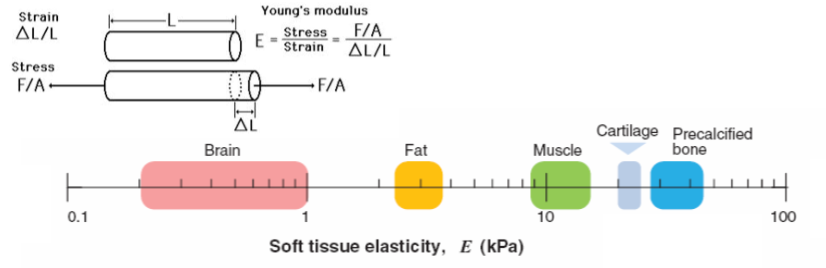
mechanical properties are matched to function of the tissue
bones need to be very stiff to carry weight
brain needs to be soft to remodel, during development
skull is stiff and protective, it allows the brain to be soft
extracellular matrix
ECM is 3d network of extracellular macromolecules found in multicellular organisms
ECM proteins such as collagen are the most common proteins in our bodies
ECM defines the mechanical properties of our tissues
mostly made of water, then ECM, most of the ECM is collagen
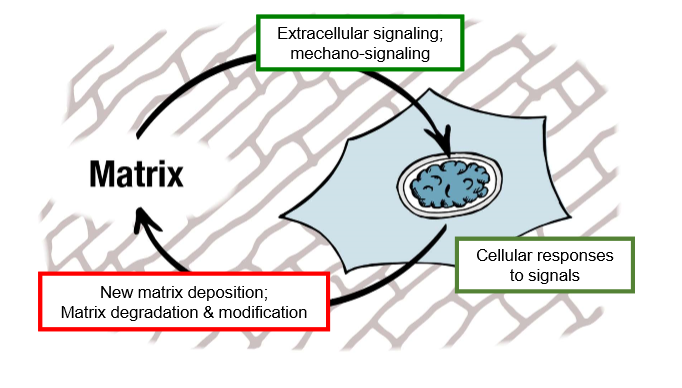
homeostatic systems are actively regulated to maintain a steady state
cells are in constant contact with ECM
signals are biochemical and cells can feel their surroundings, feel mechanical properties of their environment
secrete new proteins and remodel proteins
cells are reading signals from the environment
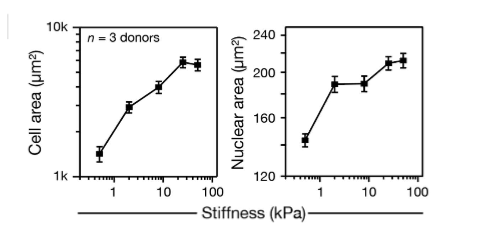
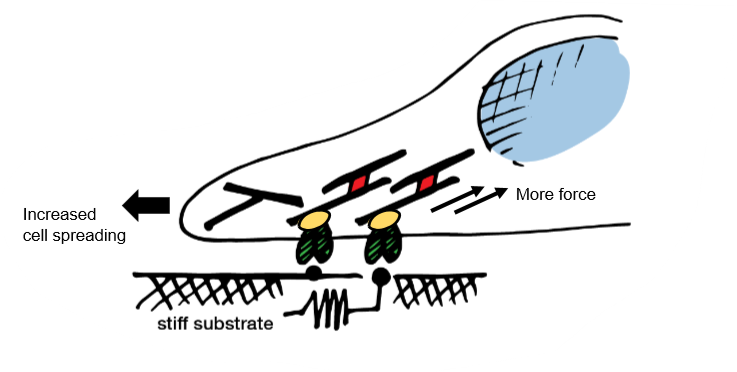
cell morphology - cells spread more on stiffer substrates
can be controlled by stiffness
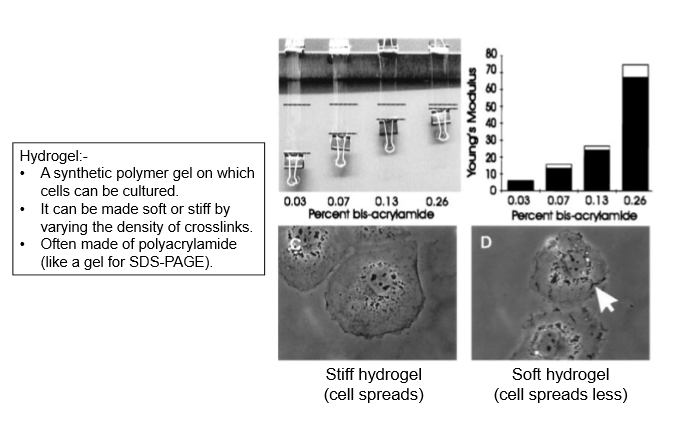
place cells on substrate, control mechanical properties of that substrate
on stiff gel, cells are bigger and spread out
cells get bigger on stiffer substrate, nuclei also becomes bigger
contractility - cells pull harder(more contractile) on stiffer substrates
cells can only feel stiffness by deforming their surroundings
able to know mechanical properties by interacting with it
experiment- cells cultured on a spring, the stiffer the spring, the harder the cell pulls against it
it reaches a plateau, a cell can only exert so much force, reached maximum
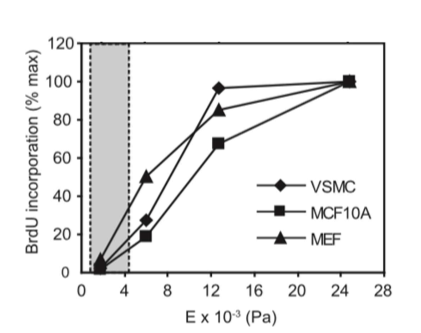
proliferation
cells grow faster on stiffer substrates
apoptosis - apoptosis is lower on stiffer substrates
cell on stiffer environment are less likely to undergo apoptosis
there is faster proliferation, so apoptosis is slower on a stiff substrate
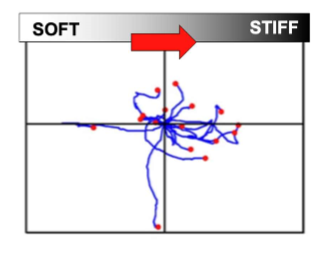
movement(durotaxis) - cells migrate towards stiffer regions
cells move from soft to stiff environment
durotaxis is the movement across a gradient o stiffness
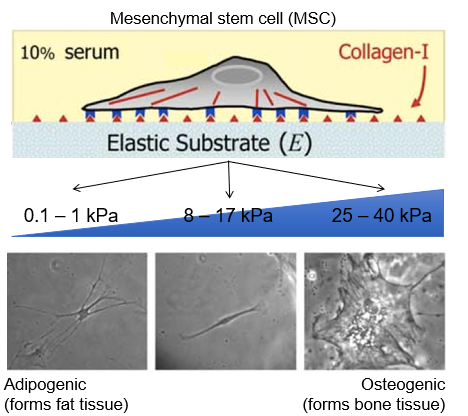
differentiation - stiffness can direct cell fate
soft substances drive differentiation to soft tissue types (e.g.fat)
stiff substrates drive differentiation to stiff tissue types (e.g.bone)
stem cells differentiate like bone on stiff environment
form adipose when on soft tissue
mechanotransduction is the conversion of a mechanical input into a biochemical signal
mechanical input converted to biochemical signalling pathway
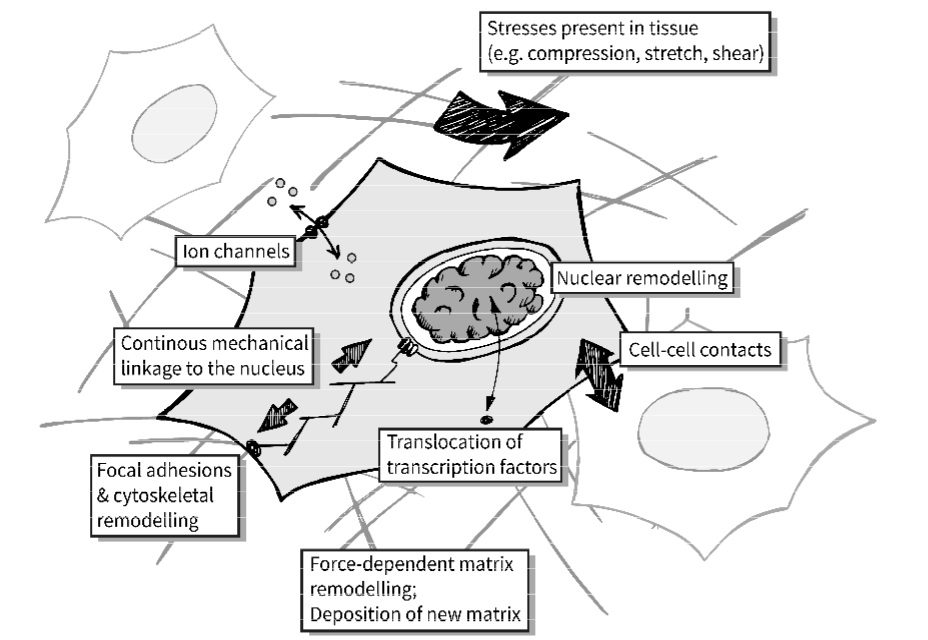
cells can only feel stiffness by deforming their surroundings
needs feedback mechanism to know if surrounding is soft or stiff
a biochemical signal leads to a pathway which can modify the protein
cells need mechanisms of
force generation - acto-myosin contraction
force transmission- cytoskeleton
mechanosensing - conversion into biochemical signals
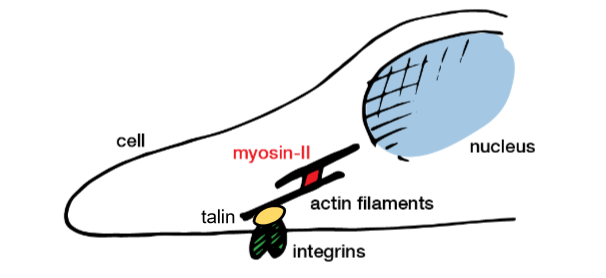
focal adhesion complex and cytoskeletal tension (i)
integrins- membrane proteins that form focal adhesion complexes that tether the cytoskeleton to the matrix
actin-polymeric filaments, major component of the cytoskeleton, growth of filaments drives cell spreading
myosins- molecular motors pull against actin filaments, causing contractility
talin- a protein that deforms when pulled on, activating a signalling cascade(conversion into biochemical signal)
formation of filaments drives cell spreading
actin pushes forwards, filaments are dissolved and pulled back- retrograde flow
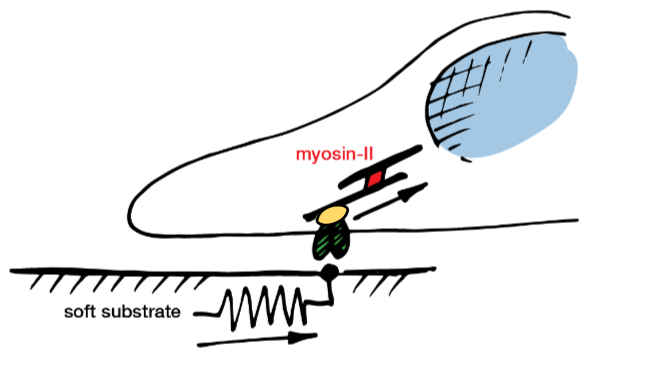
focal adhesion complex and cytoskeletal tension (ii)
cells pull on their surroundings
actin is polymerised at the edge of the cell and pulled by myosin-II
if surrounding is soft, soft surroundings will be deformed
protein unfolding releases new domains and interactions
this activates downstream signalling pathways
if stiff, proteins in the cell are deformed
binding sites are revealed, heads up signalling cascade
if substrate is stiff, signalling proteins are activated
MAPK- mitogen activated protein kinase
RhoA- transforming protein RhoA
MAPK and RhoA cause the cell to make more myosin and actin, so the cell pulls harder
increased expression of myosin leads to increased contraction
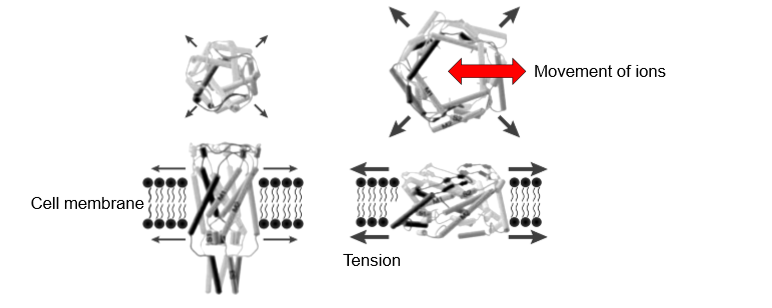
mechanosensitive ion channels
form pores
TRPV4- transient receptor potential vanilloid 4
opening of pore allows ions to enter and leave which can lead to a signalling cascade
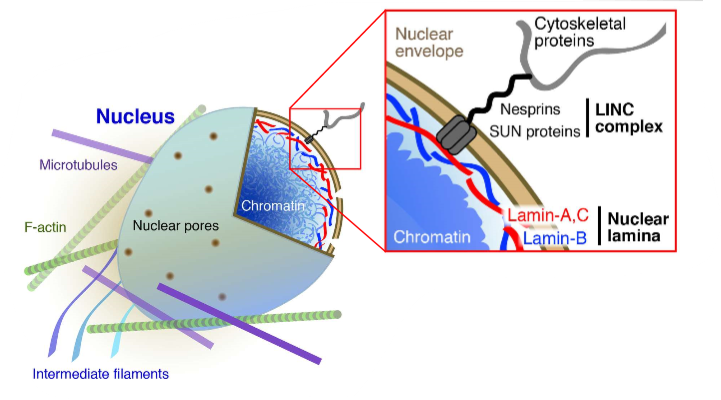
transmission of force to the nucleus
transferred into chromatin
different sites will be active
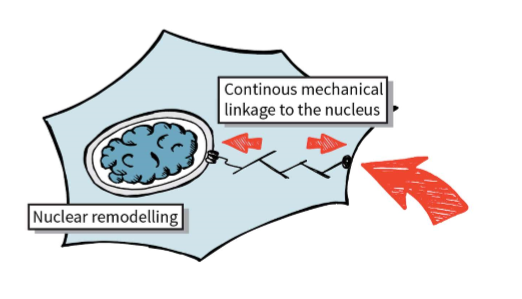
disruption of LINC complex blocks mechanotransmission to nucleus
ties cytoskeleton and cytosol to the nucleus
inside of nucleus tethers to lamina, leads to robustness to nucleus
organisation of chromatin can regulate its activity
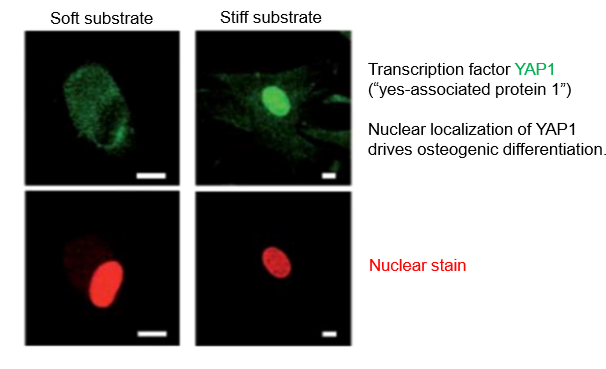
mechanosensitive translocation of transcription factors
YAP1 has roles in development and cancer
regulated by whether it’s in the nucleus
if protein is out of the nucleus, it becomes inactive because it can’t interact with DNA
moving transcription factor can be regulated mechanically
stiff environment, YAP moved in nucleus
YAP drives cell differentiation
mechanical regulation of transcription factors allows control of specific genetic programmes
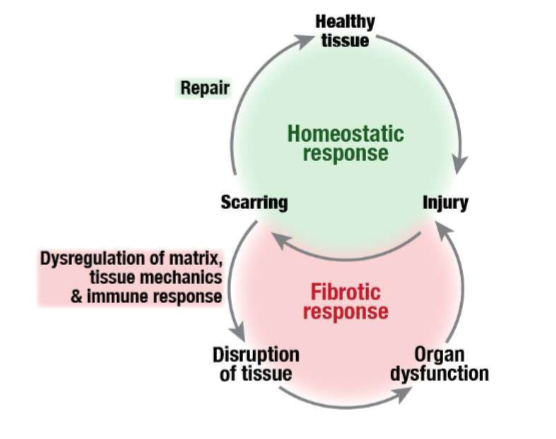
fibrosis is dysregulation of extracellular matrix
misregulation of feedback and loss of homeostasis cause cells to deposit too much matrix - firbrosis
when out of balance, too much matrix is produced
fibrotic diseases
fibrosis makes tissues stiffer
mechanical properties are no longer matched to function
tissue mechanics are matched to function
fibrotic system has too much matrix which pushes cells out of healthy position, moves on different position on the scale
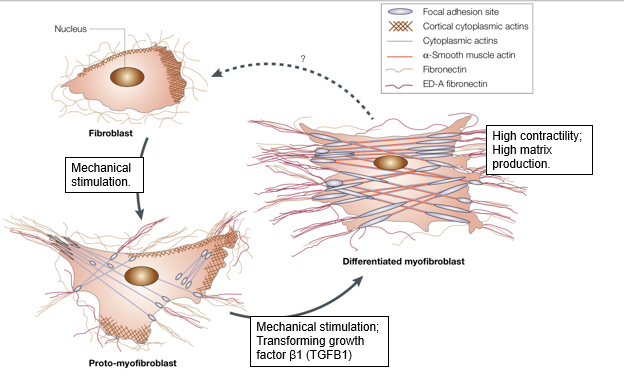
role of fibroblasts
fibroblasts are the cells responsible to synthesising extracellular matrix
fibroblasts can move towards sites of injury(e.g. durotaxis)
they are necessary for wound healing
can be cultured in 2D
can undergo durotaxis
fibroblasts can be activated at sites of injury to make myofibroblasts
myofibroblasts are more contractile and secrete more ECM
fibrotic diseases
too much matrix can compromise gas exchange or lead to too little blood
some diseases are sever atherosclerosis and COPD(chronic obstructive pulmonary disorder)
tissue repair and fibrosis
scarring can be healthy
rapidly prevents further damage
scarring is healthy response to tissue injury
too much scar tissue can lead to loss of function
excess scar tissue can restrict movement and prevent healthy function
further damage exacerbates the injury
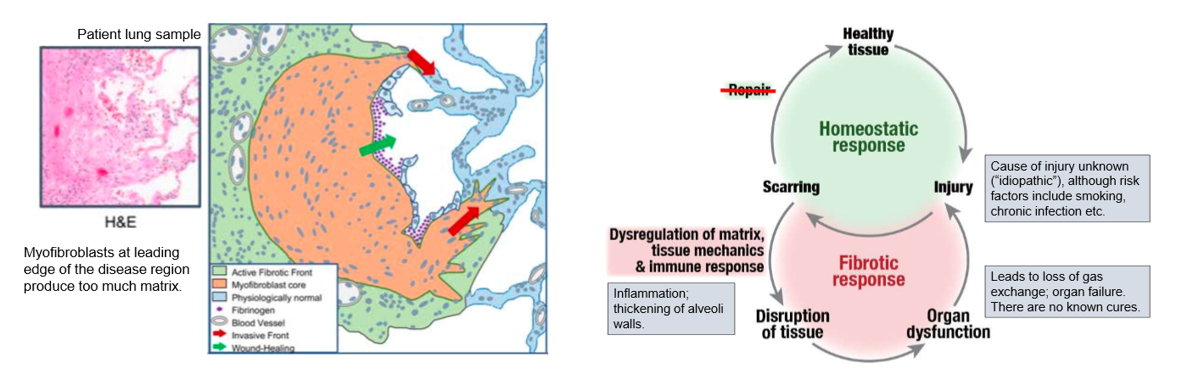
case study- idiopathic pulmonary fibrosis (i)
air sacs in thin membrane allows gas exchange
IPF is fibrosis of the alveoli, prevents gas exchange, can’t get rid of CO2
affects 14-43 per 100,000 people
seen more commonly in men
common symptoms -
shortness of breath
chronic, dry cough
finger clubbing due to growth factor signalling
occasional symptoms
fatigue
weakness
weight loss
cause is unknown but risk factors- smoking, environmental exposure, chronic viral infections, abnormal acid reflux, family history of the disease
average time to diagnosis is 1-2 years after onset of sytmptoms
50% die within 2-3 years after diagnossi
aging is a risk factor
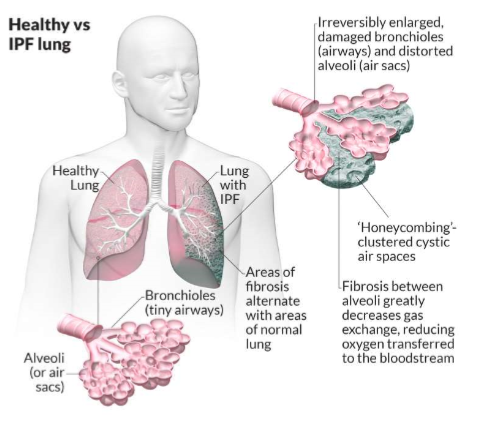
case study- idiopathic pulmonary fibrosis (ii)
build up collagen I causes stiffness and blockage of gas exchange
change in tissue mechanics
can lead to organ failure
positive feedback loop