Buda Academic Chemistry CH 5.1- Light, Energy, Wavelength, Frequency
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
electromagnetic radiation
form of energy that exhibits wavelike behavior as it travels through space
What are some example of electromagnetic radiation?
visible light, sun, microwave, etc
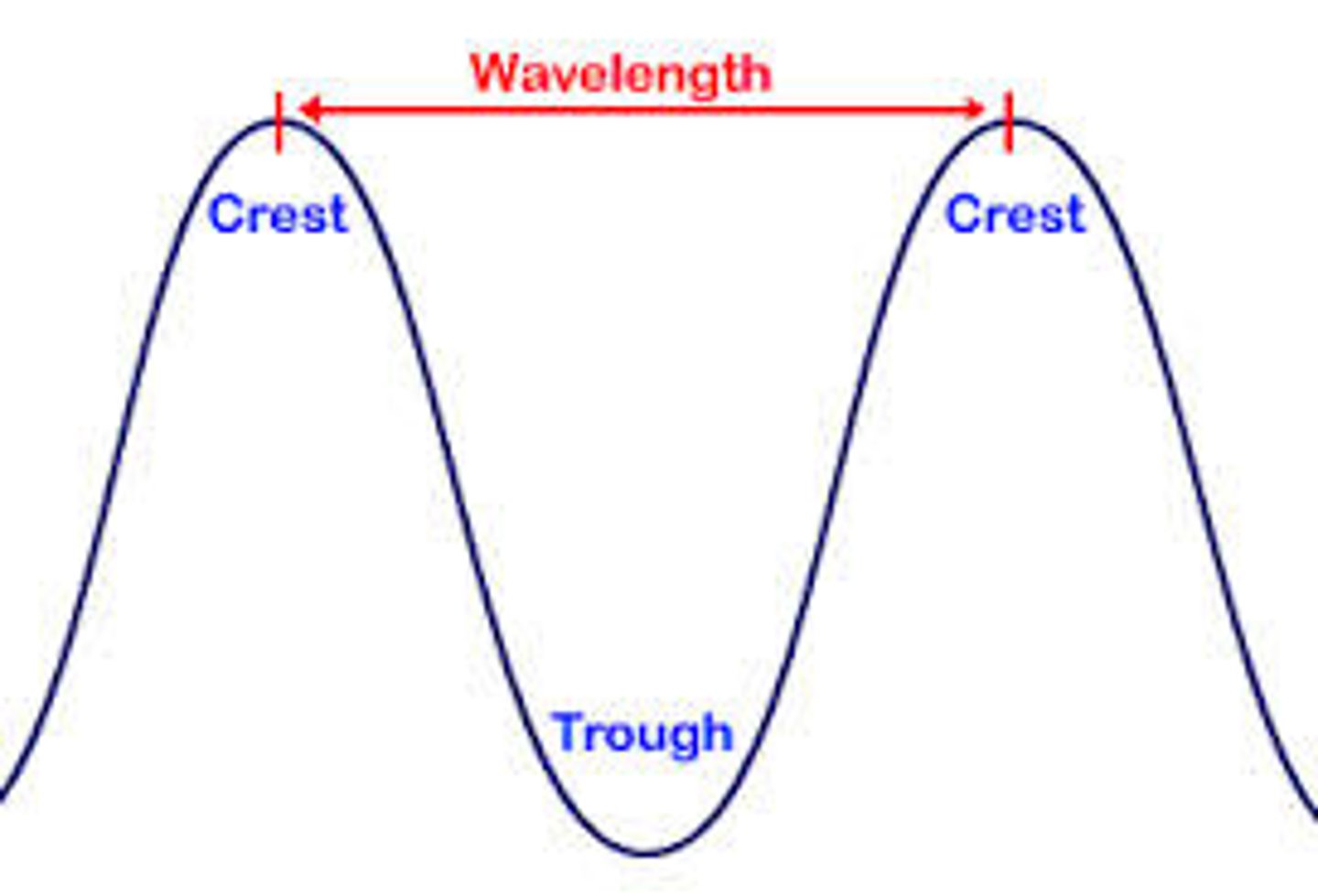
wavelength (λ - lambda)
shortest distance between equivalent points
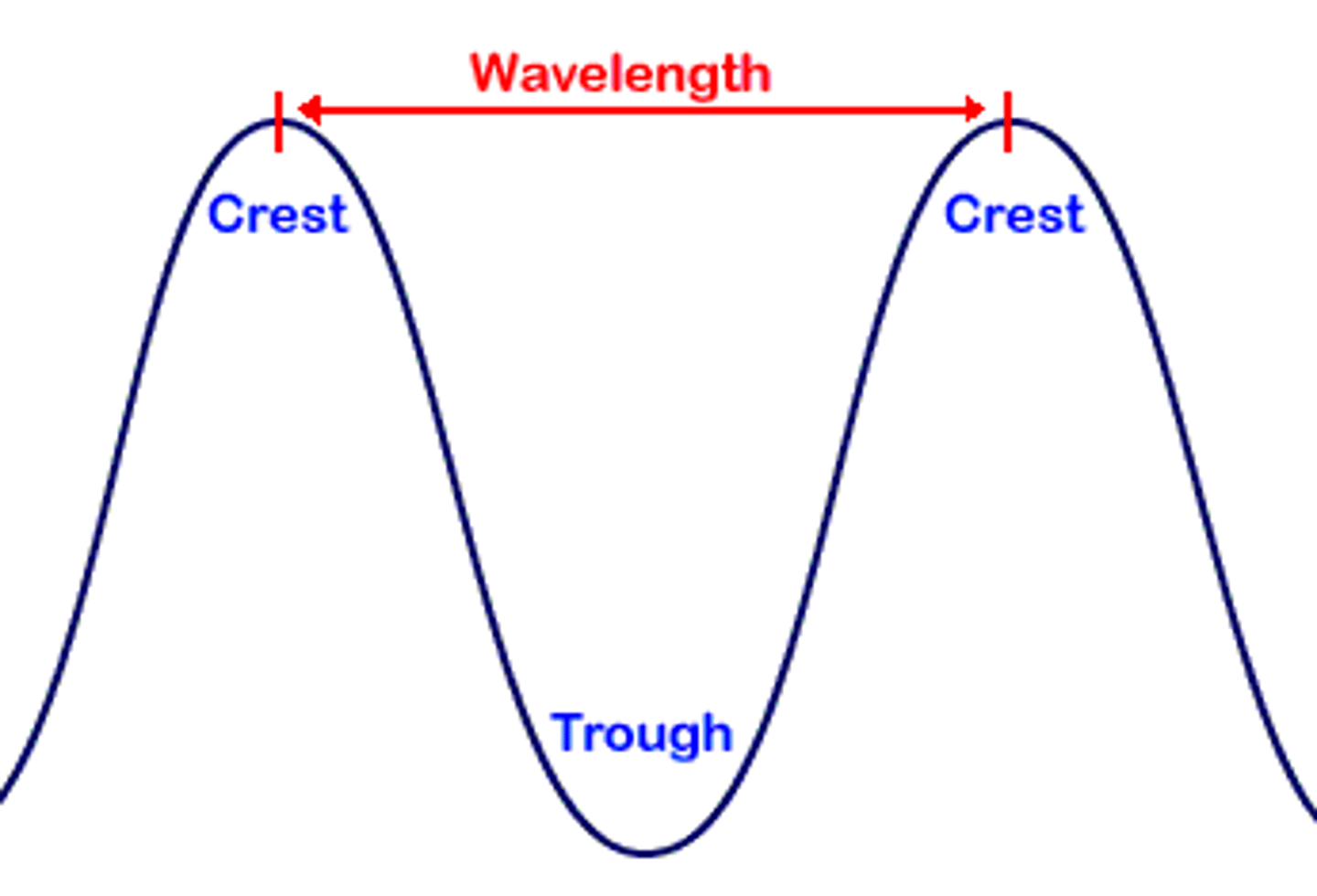
What does "λ" symbol stand for?
lambda
frequency (ν - nu)
number of waves that pass a given point per second
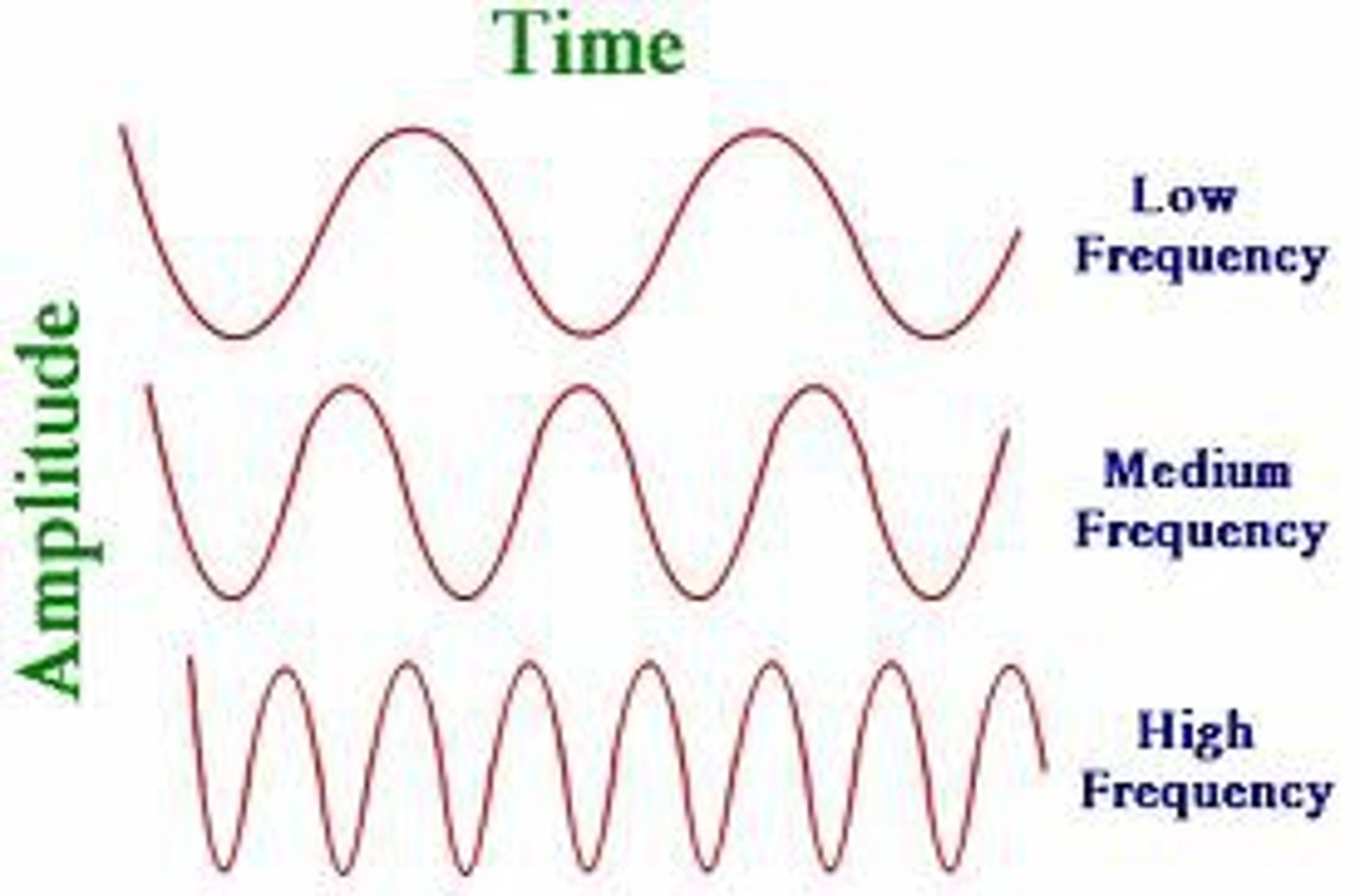
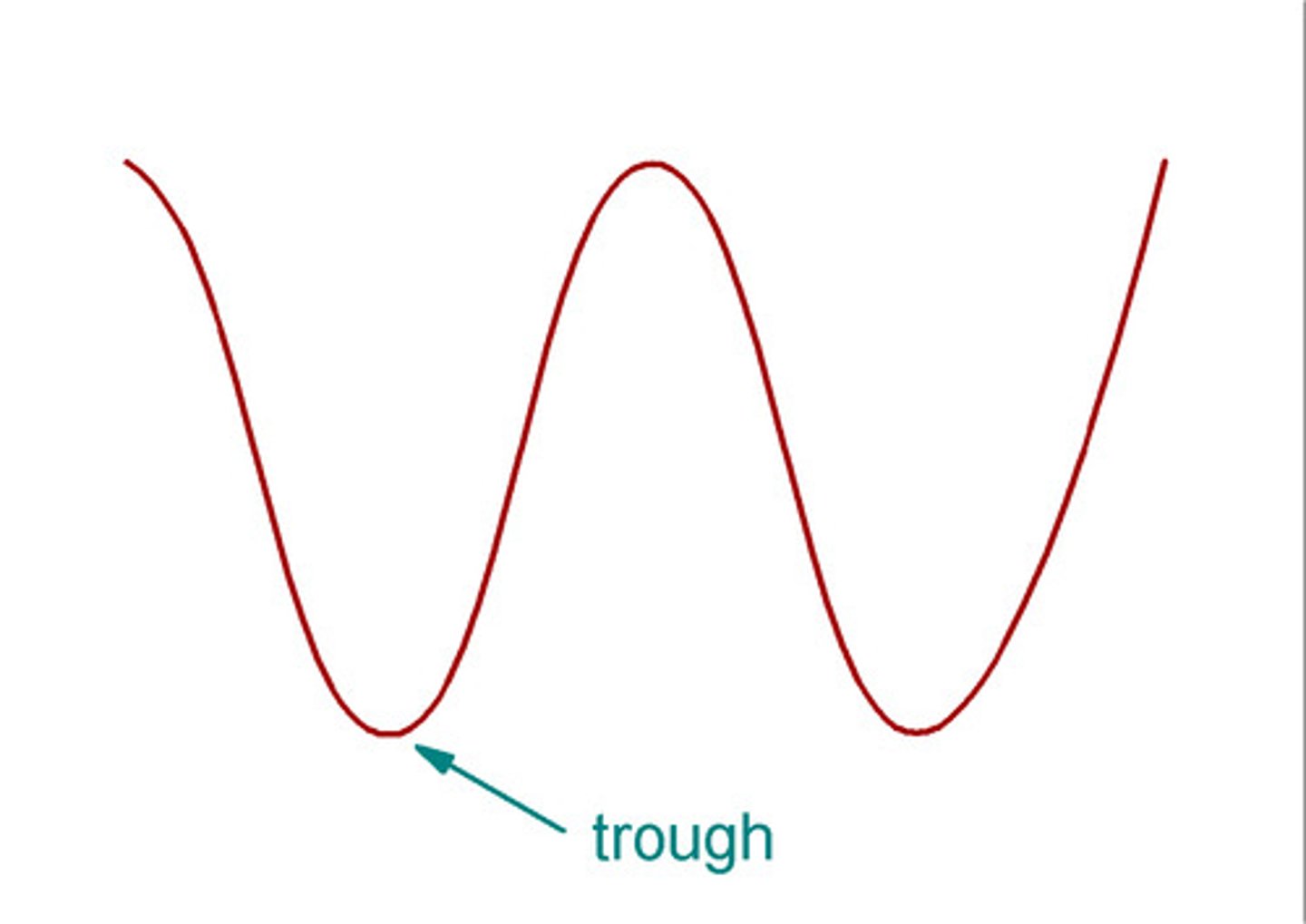
amplitude
wave's height from the origin to a crest or trough
crest
the highest point of a wave
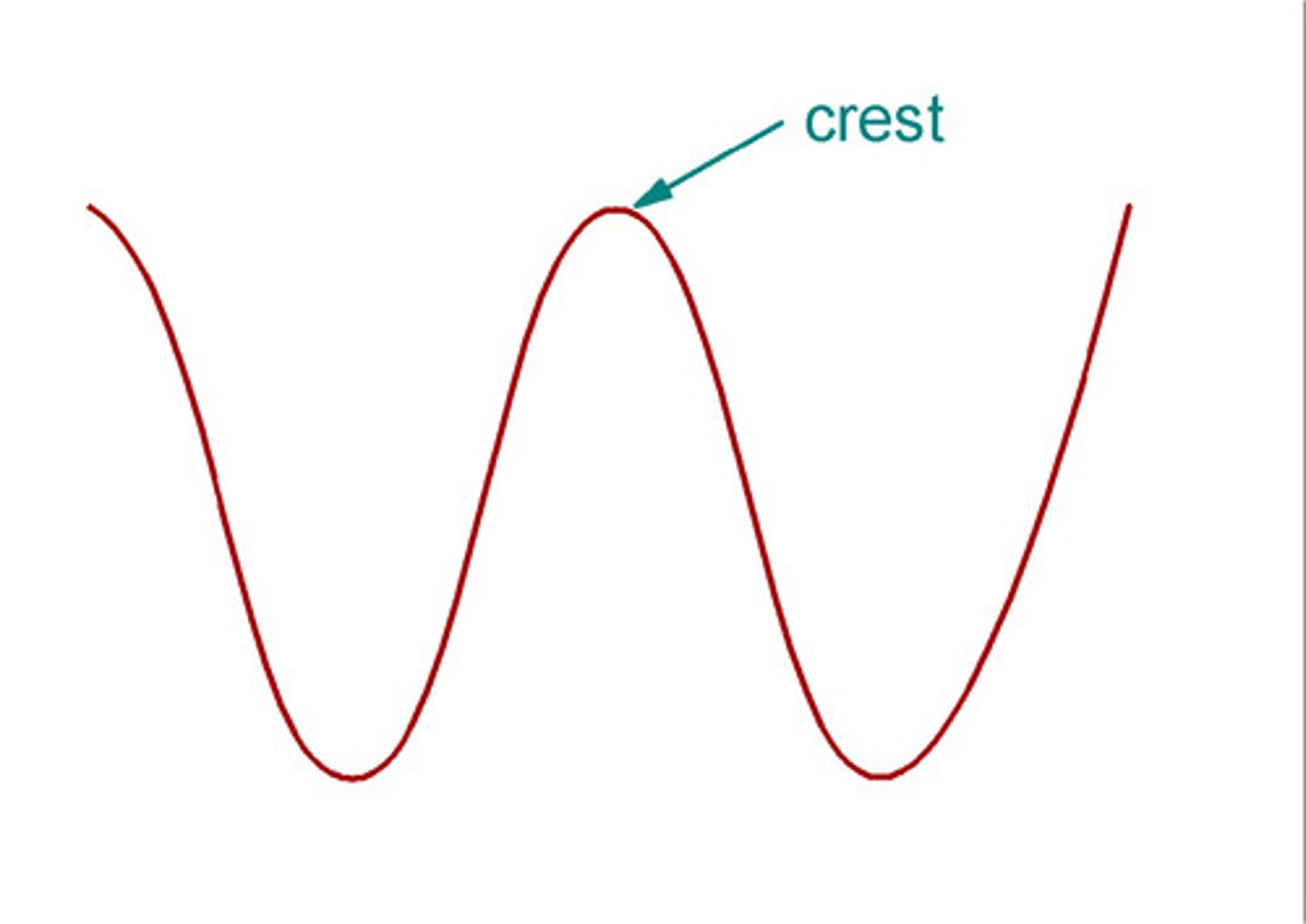
trough
the lowest point of a wave
Frequency and wavelength are inversely ___________________.
proportional
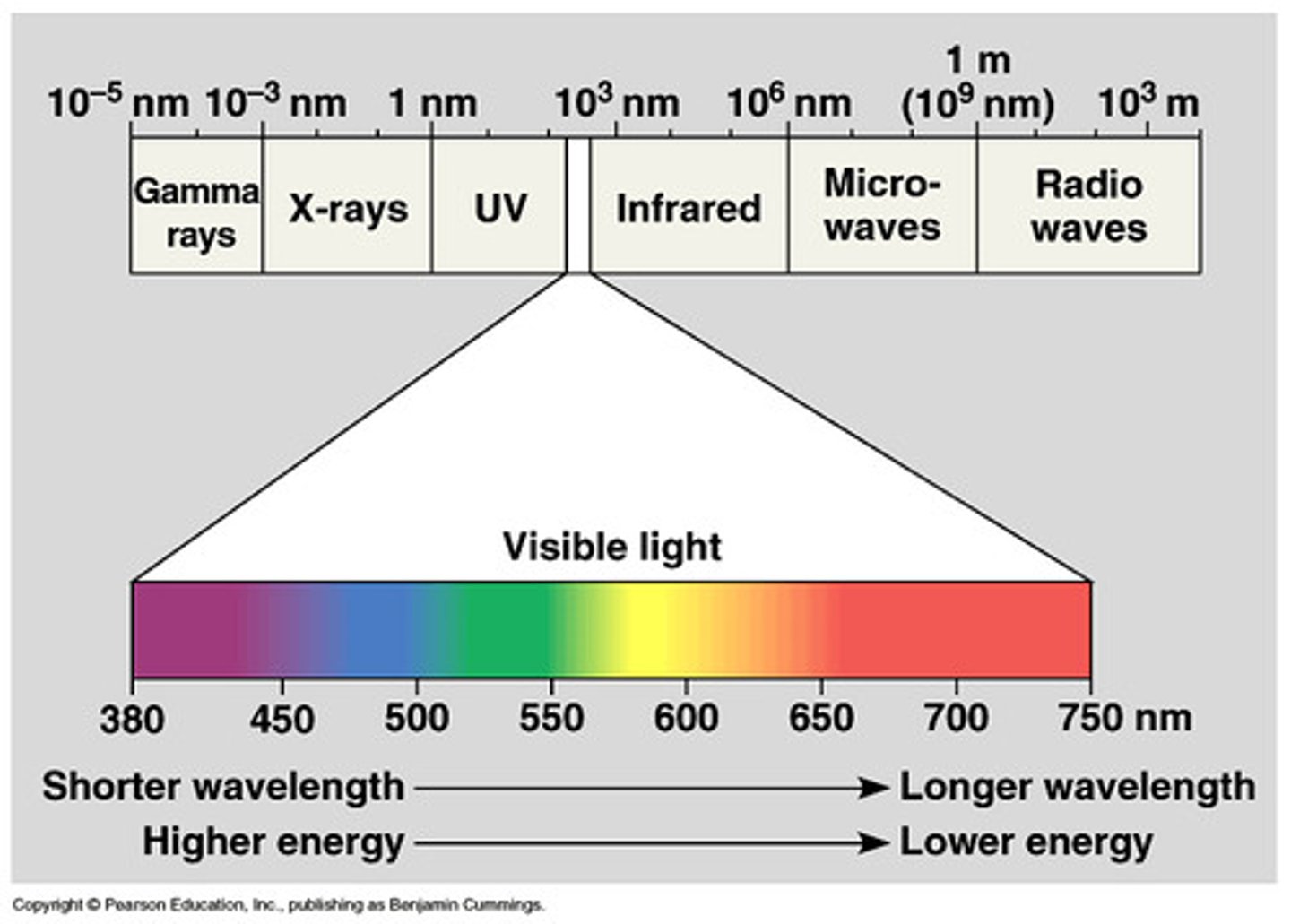
electromagnetic spectrum
encompasses all forms of electromagnetic radition
All electromagnetic waves travel at the same ________.
speed
c=λν
equation to find the speed of light
What does "c" stand for in the equation 'c=λν'?
speed of light (3.00×10^8 m/s)
What does the "λ" stand for in the equation 'c=λν'?
wavelength (m, nm, etc.)
What does the "ν" stand for in the equation 'c=λν'?
frequency (Hz, s^-1, or 1/s)
The wavelengths become (bigger/smaller) as you move down the chart (to the right).
smaller
quantum
minimum amount of energy that can be gained or lost by an atom
photoelectric effect
electrons are emitted from a metal's surface when light of a certain frequency shines on the surface
As the frequency increases on the electromagnetic spectrum, the energy (increases/decreases).
increases
What does a wave represent according to Einstein?
particle duality
What does a photon represent according to Einstein?
a massless particle that carries a quantum of energy
What are the equations for finding energy?
E=hν, E=hc/λ
What does "E" stand for in the equation for energy?
energy (J or Joules)
What does "h" stand for in the equation for energy?
Planck's constant (6.626 x 10^-34 Jxs)
What does the "v" stand for in the equation for energy?
frequency (Hz, s^-1, 1/s)
What does "c" stand for in the equation for energy?
speed of light (3.00x10^8 m/s)
What does "λ" stand for in the equation for energy?
wavelength (m)
Frequency and wavelength have a (direct/inverse) relationship.
inverse
Frequency and energy have a (direct/inverse) relationship.
direct
Practice Problem: How many Joules of energy are there in one photon of orange light whose wavelength is 630nm?
E = 3.2*10^-19 J
Practice Problem: Violet light has a wavelength of 4.10 x 10-12 m. What is the frequency?
v = 7.32*10^19 Hz
Practice Problem: Calculate the energy (E) and wavelength (λ) of a photon of light with a frequency (ν) of 6.165 x 1014 Hz.
λ = 4.86610^-7 m / E = 4.08510^-19
Conversion Factor: 1 m = _______ mm
1*10^3 mm
Conversion Factor: 1 m = ________ nm
1*10^9 nm
Conversion Factor: 1 kHz = _________ Hz
1000 Hz
Conversion Factor: 1 MHz = _________ Hz
1*10^6 Hz
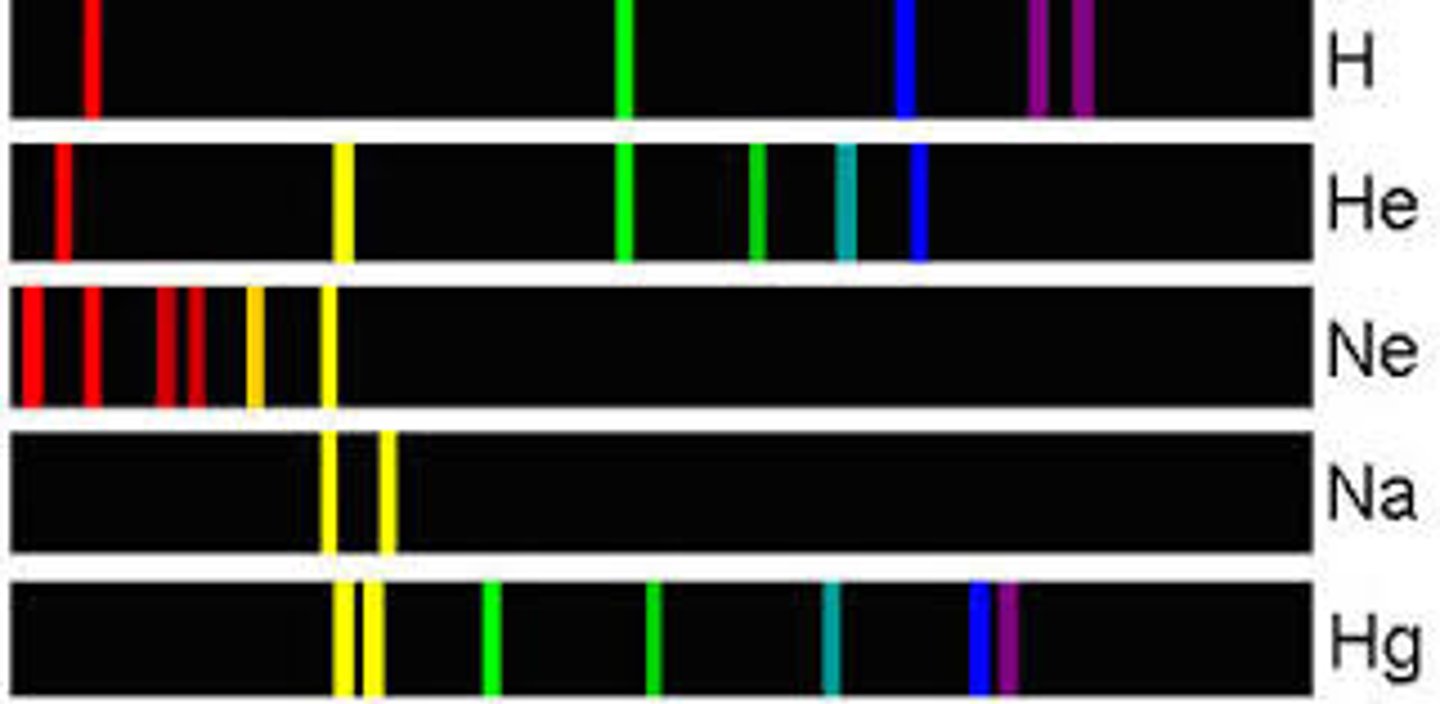
atomic emission spectrum
a set of frequencies of electromagnetic waves given off by atoms of an element; consists of a series of fine lines of individual colors
ground state
The lowest energy state of an atom
excited state
when an atom absorbs energy, its electrons move to a higher energy level
wavelength
Horizontal distance between the crests or between the troughs of two adjacent waves