Biochemistry CH 17
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Where does the citric acid cycle take place in the cell?
A) cytosol
B) mitochondria
C) nucleus
D) endoplasmic reticulum
E) lysosome
B) mitochondria
What type of enzymes are tightly associated with FAD or FMN?
A) transferases
B) cytochrome P450 enzymes
C) metalloenzymes
D) flavoproteins
E) None of the answers is correct.
D) flavoproteins
What is the enzyme-bound intermediate in the conversion of citrate and isocitrate?
A) oxalosuccinate
B) dihydrocitrate
C) cis-aconitate
D) hydroxyethyl-TPP
E) fumarate
C) cis-aconitate
Where in the cell is succinate dehydrogenase located?
A) inner mitochondrial membrane
B) outer mitochondrial membrane
C) mitochondrial matrix
D) intermembrane space
E) cytosol
A) inner mitochondrial membrane
Which of the following citric acid cycle intermediates is found at the traditional start and finish of the cycle?
A) citrate
B) isocitrate
C) -ketoglutarate
D) succinate
E) oxaloacetate
E) oxaloacetate
Which of the following is considered a product of the citric acid cycle?
A) acetate
B) -ketoglutarate
C) NAD+
D) oxaloacetate
E) carbon dioxide
E) carbon dioxide
What cycle allows for the net synthesis of glucose from acetyl-CoA?
A) Calvin cycle
B) glyoxylate cycle
C) C4 pathway
D) urea cycle
E) None of the answers is correct.
B) glyoxylate cycle
Which of the following is toxic due to its ability to react with neighboring sulfhydryls of dihydrolipoyl groups, thus blocking their reoxidation to lipoamide?
A) fluoroacetate
B) carbon monoxide
C) arsenite
D) rotenone
E) cyanide
C) arsenite
Which of the following is the primary mode of regulation for the pyruvate dehydrogenase complex?
A) feedback inhibition by citrate
B) feed-forward activation by glucose
C) allosteric activation by ATP and NADH
D) allosteric inhibition by ADP and CO2
E) inhibition by phosphorylation
E) inhibition by phosphorylation
When intermediates of the citric acid cycle are depleted by biosynthetic pathways, _____ reactions are used to replenish the citric acid cycle intermediates.
A) anaplerotic
B) amphibolic
C) amphoteric
D) biosynthetic
E) None of the answers is correct.
A) anaplerotic
The citric acid cycle is also known as the
A) Krebs cycle.
B) Cori cycle.
C) tricarboxylic acid cycle.
D) acetate cycle.
E) Krebs cycle and tricarboxylic acid cycle.
E) Krebs cycle and tricarboxylic acid cycle.
What molecule initiates the citric acid cycle by reacting with oxaloacetate?
A) pyruvate
B) acetyl CoA
C) oxaloacetate
D) glucose
E) None of the answers is correct.
B) acetyl CoA
What enzyme(s) is (are) responsible for the following reaction?
Pyruvate + CoA + NAD+ acetyl CoA + NADH + H+ + CO2
A) acetyl CoA synthetase
B) pyruvate decarboxylase
C) pyruvate dehydrogenase complex
D) pyruvate carboxylase
E) None of the answers is correct.
C) pyruvate dehydrogenase complex
What are the steps involved (in order) in the conversion of pyruvate to acetyl-CoA?
A) decarboxylation, oxidation, transfer to CoA
B) decarboxylation, transfer to CoA, oxidation
C) oxidation, decarboxylation, transfer to CoA
D) oxidation, transfer to CoA, decarboxylation
E) None of the answers is correct.
A) decarboxylation, oxidation, transfer to CoA
Which of the following vitamins are precursors to coenzymes that are necessary for the formation of acetyl CoA from pyruvate?
A) thiamine, riboflavin, niacin, lipoic acid, and pantothenic acid
B) thiamine, riboflavin, niacin, lipoic acid, pantothenic acid, and biotin
C) thiamine, riboflavin, niacin, and biotin
D) thiamine, riboflavin, and folic acid
E) None of the answers is correct.
A) thiamine, riboflavin, niacin, lipoic acid, and pantothenic acid
Which of the following functions as a "flexible swinging arm" when it transfers the reaction intermediate from one active site to the next?
A) FAD
B) NAD+
C) lipoamide
D) thiamine pyrophosphate
E) coenzyme A
C) lipoamide
Formation of citrate from acetyl CoA and oxaloacetate is a(n) _________ reaction.
A) oxidation
B) reduction
C) condensation
D) ligation
E) None of the answers is correct.
C) condensation
What is/are the chemical change(s) involved in the conversion of citrate into isocitrate?
A) hydration followed by dehydration
B) dehydration followed by hydration
C) oxidation followed by reduction
D) reduction followed by oxidation
E) None of the answers is correct.
B) dehydration followed by hydration
In which reaction is GTP (or ATP) directly formed in the citric acid cycle?
A) conversion of succinyl CoA to succinate
B) decarboxylation of -ketoglutarate
C) conversion of isocitrate to -ketoglutarate
D) condensation of acetyl-CoA and oxaloacetate
E) None of the answers is correct.
A) conversion of succinyl CoA to succinate
Which of the following enzymes are found in the glyoxylate cycle but not in the citric acid cycle?
A) malate synthase and isocitrate dehydrogenase
B) glyoxylate synthase and malate synthase
C) isocitrate lyase and malate dehydrogenase
D) malate synthase and isocitrate lyase
E) succinyl CoA synthetase and glyoxylate synthase
D) malate synthase and isocitrate lyase
Which of the following conditions will activate pyruvate dehydrogenase kinase, which catalyzes the phosphorylation and inactivation of E1 in the pyruvate dehydrogenase complex?
A) elevated concentrations of NADH and ATP
B) elevated concentrations of NAD+ and ADP
C) Ca2+
D) glucagon
E) elevated concentrations of coenzyme A
A) elevated concentrations of NADH and ATP
Approximately how many ATP or GTP equivalents will be produced from energy molecules produced during one turn of the citric acid cycle?
A) 10
B) 6
C) 9
D) 12
E) None of the answers is correct.
A) 10
In addition to pyruvate dehydrogenase, which of the following enzymes is a key regulatory site in the citric acid cycle?
A) malate dehydrogenase
B) -ketoglutarate dehydrogenase
C) succinyl CoA synthetase
D) succinate dehydrogenase
E) None of the answers is correct.
B) -ketoglutarate dehydrogenase
The glyoxylate cycle enables plants to survive using only
A) pyruvate.
B) acetate.
C) oxaloacetate.
D) lactate.
E) None of the answers is correct.
B) acetate.
Give the net equation of the citric acid cycle.
Acetyl-CoA + 3 NAD+ + FAD + GDP + Pi 2 CO2 + 3 NADH + 3 H+ + FADH2 + GTP + CoA
Why is the isomerization of citrate to isocitrate a necessary step of the citric acid cycle?
Citrate is a tertiary alcohol that cannot be oxidized. The isomerization converts the 3°
alcohol into isocitrate, which is a 2° alcohol that can be oxidized.
List the five coenzymes that are required for the oxidative decarboxylation of pyruvate
and α-ketoglutarate, and give the essential nutrient (vitamin) that is required for each.
1. Thiamine pyrophosphate: thiamine, vitamin B1
2. Lipoamide: lipoic acid
3. NAD+: niacin
4. FAD: riboflavin, vitamin B2
5. Coenzyme A: pantothenic acid
Explain why a GTP is energetically equivalent to an ATP in metabolism.
The enzyme nucleoside diphosphokinase reversibly transfers a phosphoryl group from
GTP to ADP according the reaction:
GTP + ADP GDP + ATP Conversely, a phosphoryl group can be transferred from ATP to a GDP forming GTP.
Give the reaction in the citric acid by which the energy is conserved in the formation of a phosphoanhydride bond by substrate level phosphorylation. Give the name of the enzyme that catalyzes this reaction and give the structures of the reactants and products of this reaction.
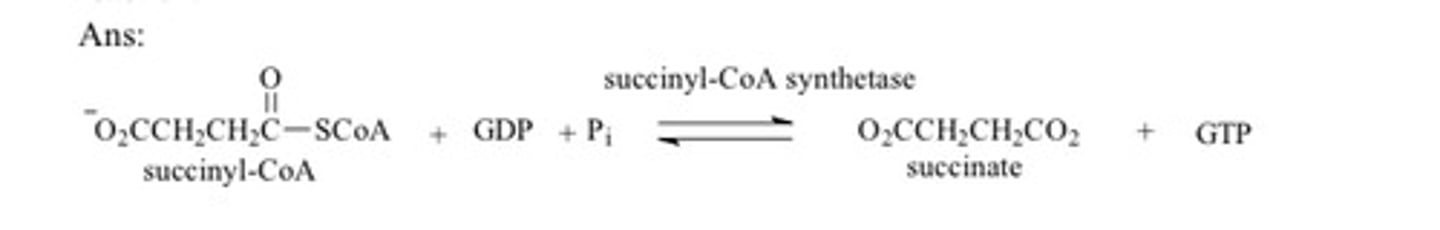
Why is it necessary that there be a mechanism to replenish oxaloacetate?
During periods of biosynthesis, oxaloacetate may be converted to amino acids for protein synthesis. Even if acetyl-CoA levels are high, the citric acid cycle will operate at reduced levels until new oxaloacetate is formed.
Starting with oxaloacetate in the glyoxylate cycle, identify what molecules enter and exit
the glyoxylate cycle.
The cycle begins with the condensation of acetyl CoA and oxaloacetate to form citrate
resulting in a net two carbon entry into the cycle. When isocitrate is hydrolyzed to succinate and
glyoxylate, succinate leaves the cycle to serve as a biosynthetic intermediate in other pathways.
Glyoxylate then condenses with another acetyl CoA to form malate, allowing for another two carbon fragment to enter the cycle.
What is the energy source that drives the condensation of oxaloacetate and acetyl CoA to
produce citrate?
Citrate synthase catalyzes the condensation of acetyl CoA and oxaloacetate to form citryl
CoA. This reaction is easily reversible. The hydrolysis of the thioester of citryl CoA forms citrate and regenerates the CoA. The hydrolysis of the high energy thioester drives the reaction toward citrate.
How does the decarboxylation of -ketoglutarate resemble that of pyruvate decarboxylation?
Both are -ketoacids, which are decarboxylated, and involve formation of a thioester
with CoA, which has high transfer potential. The enzymatic complexes and mechanisms are
similar, and the dihydrolipoyl dehydrogenase components are identical.
How many ATP equivalents are produced from the total oxidation of one pyruvate to three CO2?
The total oxidation of one pyruvate by pyruvate dehydrogenase and the citric acid cycleproduces four NADH, one FADH2, and one GTP. There are 2.5 ATPs produced when two electrons are transferred from NADH to oxygen by the electron transport chain. There are 1.5
ATPs produced when two electrons are transferred from FADH2 to oxygen by the electron transport chain. Energetically, a GTP is equal to an ATP. So, a total of 12.5 ATP equivalents are produced [(4 2.5) + (1.5 + 1) = 12.5].
The ΔG˚′ = 21 kJ mol-1 for the reaction catalyzed by isocitrate dehydrogenase, yet the
ΔG˚′ = +29.7 kJ mol-1 for the reaction catalyzed by malate dehydrogenase. Both of these
reactions involve the oxidation of a secondary alcohol. Give an explanation as to why the
oxidation of isocitrate is so exergonic.
The oxidation of isocitrate produces oxalosuccinate. The decarboxylation of oxalosuccinate produces CO2 gas, which essentially eliminates the reverse reaction. The conversion of malate to oxaloacetate does not produce CO2 and is endergonic. In contrast, the loss of CO2 makes the conversion of isocitrate to α-ketoglutarate very favorable.
How is succinate dehydrogenase unique when compared to the other enzymes in the
citric acid cycle?
It is the only enzyme embedded in the mitochondrial membrane, and it is directly associated with the electron transport chain. It is also the only enzyme that produces FADH2.
For a single rotation of the Krebs cycle, are the acetyl carbons that enter the citric acid cycle the exact same carbons that leave as CO2? Briefly explain.
No, the carbons are different. The carbons that leave as CO2 come from oxaloacetate that condensed with acetyl CoA. However, since succinate is symmetrical, and the carbons randomize, eventually all carbons are turned over.
How does the term "mad as a hatter" realistically reflect the condition?
Hatters used mercury in their craft. Frequently, the mercury would be absorbed and would react with sulfhydryls, such as those on the dihydrolipoyl groups. This would impair pyruvate dehydrogenase activity, reducing the brain's ability to metabolize glucose aerobically.
This resulted in neurological pathologies.