CH 415 Chapter 20: Molecular Mass Spectrometry
1/27
Earn XP
Description and Tags
Study of +molecular ions" M+ e- -> M+ + 2e-
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Purpose of Mass Spectroscopy
Use the difference in mass-to-charge ratio (m/z) of ionized atoms or molecules to separate them. Thus, allowing quantitation of atoms or molecules and providing structural info by the identification of distinctive fragmentation patterns.
General operation of mass spectrometer:
create gas-phase ions
separate the ions in space or time based on their m/z ratio
measure the quantity of ions of each m/z ratio
Instrumentation:
inlet → gaseous ion source (ionization) → mass analyzer (sorting of ions) → ion transducer (detection of ions) → signal processor → mass spectrum
Ionization Sources: EI, CI, FAB, MALDI, ESI
Analyzers: Quadrupoles, Time-of-Flight (TOF), magnetic sectors, Fourier transform, quadrupole ion traps
Detectors: electron multiplier, Faraday cup
Ion sources for molecular mass spectrometry
Gas-phase sources - sample is first vaporized then ionized (volatile analyses): EI (electron impact) and CI (chemical ionization)
Desorption sources - sample is converted directly into gaseous ions (non-volatile analyses): FAB (fast atom bombardment), MALDI (matrix-assisted laser desorption ionization), ESI (electrospray ionization)
Increasing Softness: (hardest) EI<CI<FAB<MALDI<ESI (softest)
Interpretation of Spectra
Ion Sources for Mass Spectrometers
Gas-Phase: EI (ionizing agent = energetic electrons) and CI (ionizing agent =reagent gaseous ions)
Desorption: FAB (ionizing agent = energetic atomic beam), MALDI (ionizing agent = laser beam), ESI (ionizing agent = high electrical field)
Energy driven process → hard ionization, soft ionization
MS with “Hard” and “Soft” Sources
A hard ionization source (EI) - shows fragments
A soft ionization source (CI) - shows parent
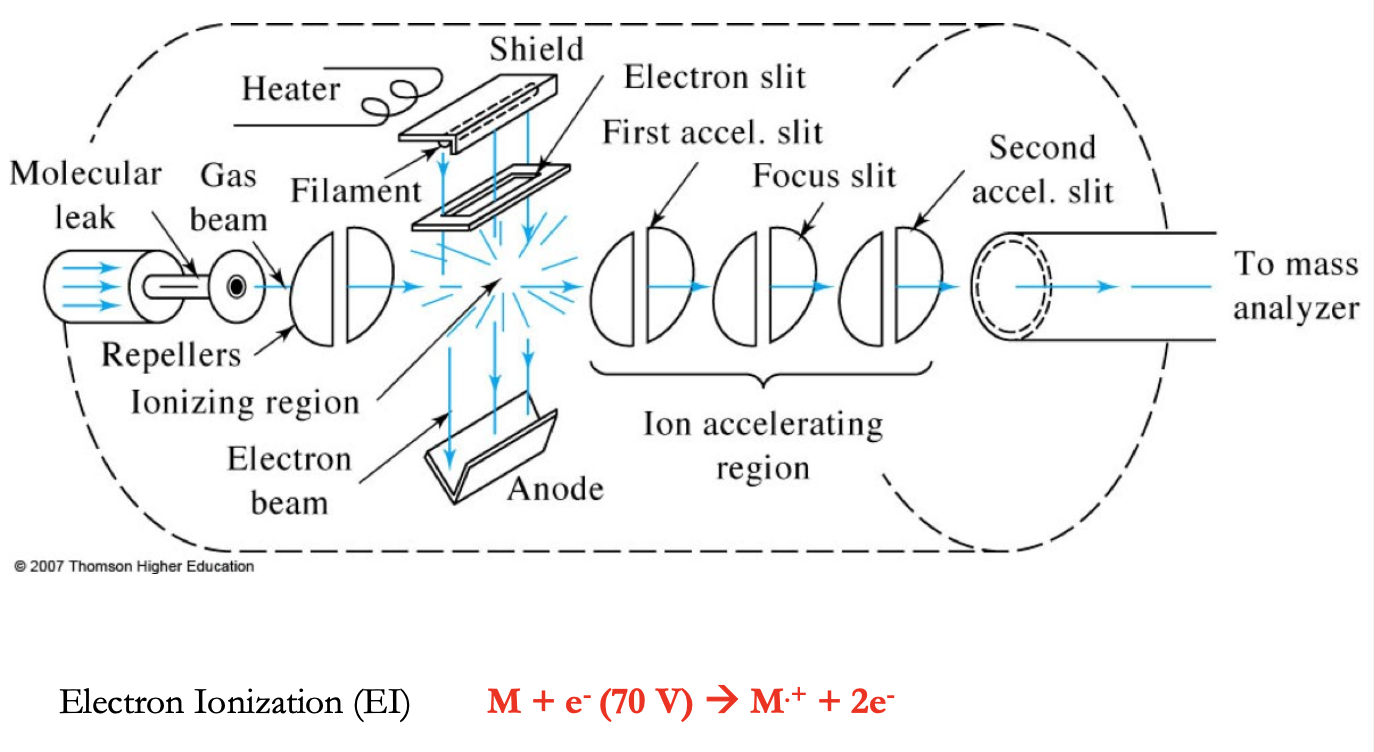
Electron Impact Source (EI)
electrons from filament hit the sample, path of electrons and molecules are on a right angle
this method is applicable to all volatile compounds (>10³ Da) and gives reproducible mass spectra with fragmentation to provide structural info.
Filament - tungsten or rhenium (our source of 70 eV e-s)
Target - anode used in association w/ the filament to produce e-s
Repeller - positively charged electrode used to “push” + ions out of the ionization source
Lens stack - series of increasingly more negative electrodes used to accelerate our ions to constant KE
Why vacuum?
-to ensure filament does not burn out
-to help vaporize samples
-to reduce collision b/t formed ions and atmospheric gases
-to remove sample from instrument after analysis
M + e- (70 eV) → M+ + 2e-
Electron Ionization Process
M (IE)→ M+. (odd-electron ion) (excess energy) → EE+ (fragment ion)
Electron removed from orbital with lowest IE n < pi< sigma
Methane (CH4 IE = 12.6 ev)
Ethene H2C=CH2 IE = 10.5 eV)
Methyl amine H3C-NH2 IE = 10.3 eV
Typical Reactions during EI
Energy = 70 eV → 6700 kJ/mol
Typical bond energies → 200 to 600 kJ/mol → EXTENSIVE FRAGMENTATION
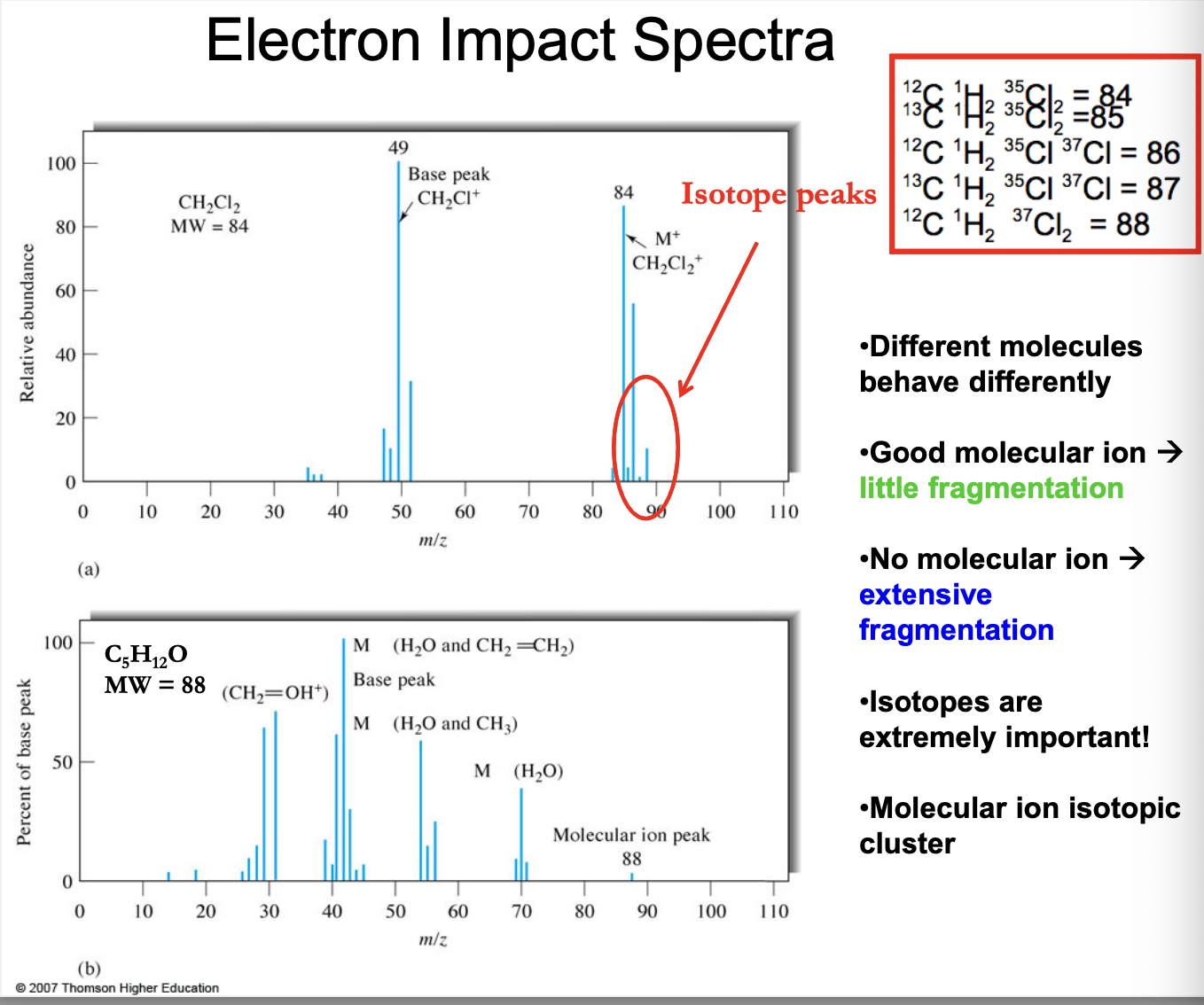
EI spectra
different molecules behave differently
Good molecular (parent) ion → little fragmentation
No molecular (parent-far right side signal?) ion → extensive fragmentation
isotopes are extremely important!
Molecular ion isotopic cluster
84 = 12CH235Cl2
85 = 13CH235Cl2
86 = 12CH235Cl37Cl
87 = 13CH235Cl37Cl
88 = 12CH237Cl2
Advantages and Disadvantages of EI Ion Sources
Advantages:
subpicomole to picomole detection limits
availability of computer data bases of over 100,000 compounds
use of fragmentation pattern as a fingerprint w/ databases to identify unknowns
Structural info obtained from fragmentation pattern
Disadvantages:
limited mass range (about 600 Da) due to thermal desorption (volatility) requirement; derivatization can extend range.
possible decomposition prior to vaporization
severe fragmentation; often resulting in no observable molecular ion
Chemical Ionization (CI)
development from EI
same compound classes as EI
gives molecular weight
Softer ionization technique
Produces M+H+ ions or M-H- ions
used to produce more abundant molecular ions when the molecule under investigation fragments using EI
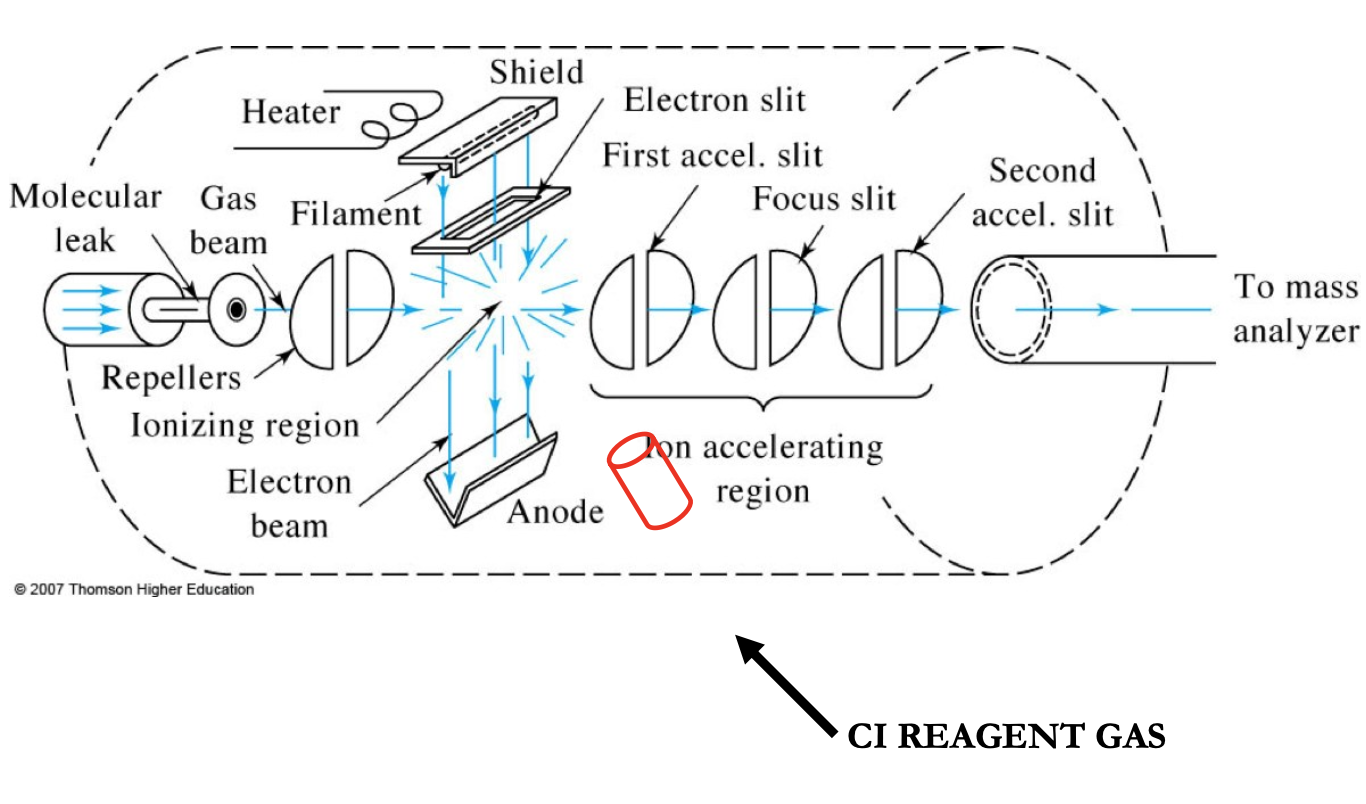
CI MS Sources
a soft ionization method
gaseous molecules of the sample are ionized by collision rxns with ions produced from a reagent gas (methane, propane, isobutane, ammonia). They produce different spectra.
It relies on our charge being transferred from a reagent molecule to our sample. Reagent ion + molecule → molecular ion + reagent ion
This method gives molecular weight info and reduced fragmentation compared to EI
Positive CI Mass Spectrometry
Reagent gases are used and after several ion-molecule rxns, produce a species BH+ which have a variety of proton affinities
Efficiency of CI: Proton affinity of analyte > proton affinity of the reagent gas
selective ionization method (ex. ammonia)
softness fo ionization depends on differences in proton affinities b/t the analyte and BH+ - large differences in PA’s results in more fragmentation
Why EI and CI (gas-phase sources) not enough?
sample must be in gas phase
not for nonvolatile or thermally unstable compounds
→ Desorption Sources
Matrix-assisted laser desorption ionization (MALDI) - soft ionization
Analyte protonation occurs (M+H+ → MH+)
analyses: polymers, proteins, DNA, large fragile molecules
matrix: large excess over analyte, absorbs UV laser light, transfer energy to analyses (common matrix is strong UV chromophore (sinapinic acid (SA))
matrix absorbs the laser light and transfer energy to analytes
analyses are desorbed, ionized (by taking proton), and accelerated to mass analyzer
MALDI matrix must:
be able to embed and isolate analyses (ex. by co-crystallization)
be soluble in solvents compatible with analyte
be vacuum stable
Absorb the laser wavelength
cause co-desorption of the analyte upon laser irradiation
Promote analyte ionization
Essential Functions of the Matrix:
isolate and encase the analyte molecules (analogous to a solvent shell). (matrix encases the analyte)
Absorb the laser energy via electronic or vibrational coupling/excitation (photons from laser excite the matrix)
Facile desorption from the condensed phase WITH the analyte molecules but WITHOUT destructive heating of the analyte molecules (“softness”)
efficient ionization of analyte molecules
MALDI is:
more tolerant of salts and complex mixture analysis than ESI
Important for huge molecules: proteins, polymers
Spectra often contain multiple charged ions
More MS Analyzers
Electrospray Ionization Source (ESI)
Excess ion charges accumulate at droplet surface evaporation → Rayleigh limit reached → Coulomb explosion (droplet fusion) → desolated ions
Rayleigh limit - electrostatic repulsion forces of ions at droplet surface equal the surface tension forces holding the droplet together
At the Rayleigh limit, surface tension can no longer support the charge leading to Coulombic explosion
Iribarne-Thompson Model
charge density increases
Rayleigh limit (Coulomb repulsion = surface tension)
Coulomb explosion (daughter driblets)
Evaporation of daughter droplets
Desorption (desolation) of ions from the droplets into the ambient gas (IONS FORMED)
ESI mass spec of myoglobin - multiple intact ion charge states
mass = 16992 Da +20 ion
m/z = 19992 + 20/ 20 = 850.6
Protein molecular weight = M = 16,951.5 Da
add n protons → m/z = (M +1.0078n)/n
m/z (at z=20) = (16,951.5 +20)/20 = 848.6
multiply charged ions result in lower m/z!
lower mass range spectrometer can be used
Advantages of ESI
allows for the direct coupling of liquid separations to mass spectrometer
multiple-charging extends the mass range of an analyzer by a factor equal to z
Soft(est) ionization technique which allows for the analysis of non-covalent complexes
“No” matrix interference
Practical mass range up to 100 kDa
good detection limits (fentomole to attomole)
More MS Analyzers
Resolution? - the capability of a MS to differential b/t masses
R = m/deltam
deltam : mass difference b/t two adjacent peaks that are just resolved (height of the valley < 10% of the peak)
M: nominal mass of the first or mean of the two peaks
Estimate accuracy of measurement if resolution is known
Ex. If R = 5000, at mass 500 range
R = m/delta m : 5000 = 500/delta m → deltam = 500/5000 = 0.10
Determine R required C2H4+ and CH2N+ have masses of 28.0313 and 28.0187
R = m/deltam = 28.025/0.0126 = 2220 (average of the two/distance b/t the two)
Sample Inlet Systems
Batch inlet systems
direct probe inlets
Chromatographic inlets
Capillary electrophoretic inlets
Inlet Systems
Gas/Liquid Inlet System
Solid/Matrix Inlet System
Mass Spectrometry Mass Analyzers
Magnetic Sector Mass analyzers
ion-trap analyzers
TOF mass analyzers
Quadrupole mass analyzers
How does an ion with a velocity vector (v) behave in a magnetic field (B)?
magnetic field (B): F = v x B
the ion undergoes a force perpendicular to velocity and magnetic field vectors, and is deflected through a circular path with radius r.
Ions w/ different m/z will be deflected with different radii of curvature
Orbits of charged particles moving in magnetic field:
qvB > mv²/r (spiral)
qvB = mv²/r (circle)
qvB = mv²/r (unbound/escapes)
Magnetic Sector Physics
KE = zeV = ½ mv²
Magnetic force = FM = Bzev
centripetal force = Fc = mv²/r
FM = Fc
Bzev = mv²/r
v=Bzer/m
m/z = (B²r²e)/2V
r= radius of curvature
path of heavier and lighters ions (they will hit the outside)
Magnetic Sector Mass analyzer
The mass spectrometer is an instrument which can measure the masses and relative concentrations of atoms and molecules. It makes use of the basic magnetic force on a moving charged particle.
After ionization, acceleration (accelerating voltage applied), and selection of single velocity particles, the ions move into a mass spectrometer region where the radius of the path and thus the position of the detector is a function of the mass (r = mv/qB = mEs/qBBs)
Ion Trajectory:
- ions from the ion source are accelerated to high velocity through a magnetic sector, in which a magnetic field is applied perpendicular to the direction of ion motion.
-Ion velocity then becomes constant but in a circular path at angles of 180, 90, or 60 deg.
-Ions are sorted mass to charge ratio by holding V and r constant while varying B (m/z = (B²r²e)/2V
Advantages:
high resolution, sensitivity, and dynamic range
high-energy CID MS/MS spectra are very reproducible
Disadvantages:
not well-suited for pulsed ionization methods (ex. MALDI)
usually larger and higher cost than other mass analyzers
Single Focusing Magnetic Sector vs. Double Focusing Mass spectrometers
Single Focusing Magnetic Sector:
ions at source with same m/z ratio
ions with diverging directional substitution will be acted upon in the same way
Brings ions with different directional orientations to focus
limits the resolution
Double Focusing Mass spectrometers:
passes ions through an electrostatic analyzer (ESA) which limits the range of the KE of ions reaching the magnetic sector
only ions with the same average kinetic energy pass through the ESA slits into the magnetic sector
Two focal planes at the ion collector (energy focal plane -takes place in electrostatic analyzer) and directional focal plane - occurs in the magnetic sector)
Increases resolution
Ion Cyclotron Resonance Mass Analyzer
Ion Cyclotron Resonance:
r = mv/zeB
wc = zeB/m
FT-MS (Ion cyclotron):
ions are arrested in cell inside a static magnetic field
ions will move in a circular trajectory radius given by: r = mv/zeB
the corresponding cyclotron frequency is: wc = zeB/m
First two are the ion cyclotron resonance phenomenon:
ions before excitation. They are in their natural cyclotron radius within the magnetic field.
Ions during excitation with a radio frequency. This excites the ions to a larger cyclotron radius
Ions after excitation. The cyclotron radius remains in its larger state
Detection of image current:
independent of B0 strength
increases linearly with r and with ion charge