Physics: Unit 11 Ideal Gas Laws
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Can a solid:
be easily compressed?
does it have a definitive shape?
and does it have a definitive volume?
No, yes, yes
Can a Liquid:
be easily compressed?
does it have a definitive shape?
and does it have a definitive volume?
No, no, yes
Can a Gas:
be easily compressed?
does it have a definitive shape?
and does it have a definitive volume?
Yes, No, no
Describe the motion of particles in solids.
Particles vibrate in fixed arrangements
Describe the motion of particles in liquids.
Particles vibrate, rotate and translate and are able to swap places with it’s neighbour.
Describe the motion of particles in gases.
Particles vibrate, rotate and translate fast, freely and randomly. They fill it’s container and are in a straight line.
Why are gases easy to compress?
They are far apart and intermolecular forces between particles are weak.
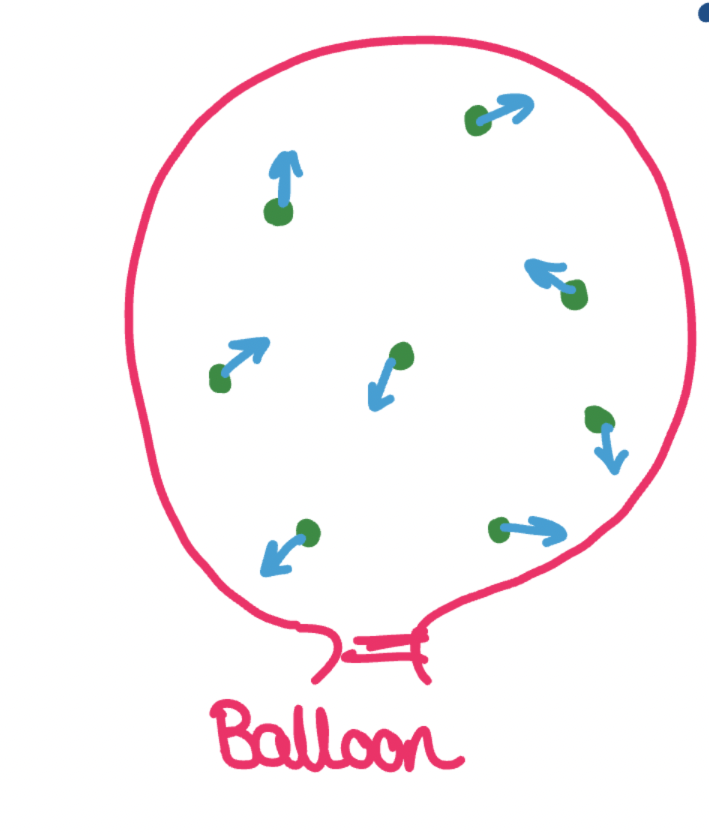
How does a gas create pressure (eg balloon)?
Gas particles move randomly at high speeds
They collide with the walls of their container (also each other)
Each collision causes a force on the walls.
The force acts in all directions over the surface area of the balloon.
A force over an area is a pressure!
p = F/A
How does increasing number of particles in the container affect pressure caused by gas?
Increases no. of collisions → increases force on walls → increases pressure.
How does increasing the size if the container affect pressure caused by gas?
Decreases no. collisions → Decreases force on walls → decreases pressure.
What is Boyles law?
(In a closed container) Volume is inversely proportional to pressure when the temperature is kept constant, meaning as volume decreases, pressure increases.
P1V1 = P2V2
Describe the practical for Boyles law.
Set up apparatus as shown
Take readings of gas volune abd pressure
Open the valve
Use level to activate bicycle pump.
Close valve
Repeat steps 2-5 to get range of values
Multiply the results.

What two quantities must be kept constant in the investigation?
Amount of gas and temperature.
What happens to gas particles when you increase the temperature?
Particles move faster because they have more kinetic energy
More collisions → more force → more pressure.
Opposite is also true: Decreased temperature = Decreased particle energy = Decreased pressure
What is absolute zero?
When the particles have zero kinetic energy and stopped moving. It is the lowest possible temperature
Has a value of -273°C
What is the kelvin temperature scale?
Temperature in Kelvin tells us how much energy the particles have.
0 Kelvin is -273°C and change of 1°C = change of 1K
What is the relationship between pressure and temperature?
P1/T1 = P2/T2
TEMPERATURE MUST BE IN KELVIN