Pancreatic Hormones and Antidiabetic Drugs
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
86 Terms
Antidiabetic Drugs
Insulin
Sulfonylureas
Meglitinides
Biguanides
Thiazolidinediones
A-Glucosidase Inhibitors
GLP-1 Agonists
Dipeptidyl Peptidase 4 inhibitors
Amylin Analogs
SGLT-2 Inhibitors
Polypeptide hormone produced by the pancreatic beta cell
insulin
Insulin synthesis and release are modulated by the following proponents and pathways:
proponents:
Glucose
Amino acids, fatty acids, and ketone bodies
Islets of Langerhans
pathways:
a-adrenergic pathways
B-adrenergic stimulation
Elevated intracellular Ca2+ acts
– most important stimulus for insulin synthesis and release
Glucose
pathway that inhibit secretion of insulin – the predominant inhibitory mechanism
a-adrenergic pathways
pathway that increases insulin release
B-adrenergic stimulation
MOA:
Binds to the extracellular domain of specific high-affinity receptors (with tyrosine kinase activity) on the surface of liver, muscle, and fat cells.
specific tyrosine residues of the insulin receptor become phosphorylated which will lead to a signal transduction cascade.
Insulin
Actions of insulin for K+, liver, and muscles
Insulin promoted systemic cellular K+ uptake
Liver: inhibits glucose production
Muscle: Increase glycogen deposition
how are insulin preparations classified
by the timing of its action in the body, including the onset of action and duration of action
Indications of insulin preparations:
used to treat all manifestations of hyperglycemia in both type 1 (insulin-dependent) and type 2 (non-insulin dependent) diabetes mellitus
Adverse effects of insulin preparations
hypoglycemia: symptoms include tachycardia, tremor, sweating, confusion, agitation, and in more severe cases, loss of consciousness or coma
Hypokalemia, hypertrophy of the SC fat at the injection site, weight gain
Rapid-acting onset, peak and duration, and directions for use?
10-30mins; 30-90mins; 3-5hrs
directions: Usually taken immediately before a meal to cover the blood glucose elevation from eating ; Often used with longer-acting insulin
examples of rapid-acting insulin preparations?
GluLisAsp
Insulin aspart, Insulin glulisine, Insulin lispro
Short-acting onset, peak and duration, and directions for use?
30-60mins; 2-4 hrs; 6-12hrs
Directions: Usually taken immediately before a meal to cover the blood glucose elevation from eating ; Often used with longer-acting insulin
example of short-acting insulin prep?
Regular Insulin
Intermediate-acting onset, peak and duration, and directions for use?
1-3hrs ; 4-8hrs; 12-16hrs
Directions for use: Often combined with rapid-or short acting insulin
example of intermediate-acting insulin prep?
Insulin NPH
Long-acting onset, peak and duration, and directions for use?
1-2hrs; Minimal; Up to 24 hrs;
Directions: Often combined with rapid-or short acting insulin
insulin preparations that lower blood glucose levels when rapid-acting insulin stops working
Long-acting insulin preparations
(Insulin determir ; Insulin glargine)
Ultra-long acting insulin preparations
(Insulin degludec)
examples of long-acting insulin preparations:
GlaDeter
Insulin determir Insulin glargine
Ultra-long acting onset, peak and duration, and directions for use?
Not available; Minimal; Up to 42 hrs
Directions: Often combined with rapid-or short acting insulin ; they lower blood glucose levels when rapid-acting insulin stops working
These agents are modified with different amino acid residues to make them more soluble, allowing them to rapidly dissociate into monomers
They are often injected minutes before a meal and provide better postprandial control of glucose levels than regular insulin
Rapid-acting insulin preparations
Regular crystalline insulin naturally self-associates into a hexameric molecule (6 insulin molecules) when injected SQ. before it is absorbed, it must dissociate to dimers and then to monomers
Short-acting insulin preparations
This insulin is modified with the addition of protamine, which prolongs the time required for absorption and increases the duration of action
Intermediate-acting insulin preparations
Insulin NPH, NPH stands for?
neutral protamine Hagedorn
These agents were modified to mimic basal insulin secretion and have a steady release with no peak effect
Long-acting
Dosing considerations of insulin preparations
(and why?)
Patients with type 2 DM may require higher doses of insulin, due to insulin resistance - presence of honeymoon phase in T1DM patients
may occur in patients recently diagnosed with Type 1 DM
It occurs when beta cells in the pancreas can still secrete enough endogenous insulin to aid in blood glucose control, resulting in reduced exogenous insulin requirement
Honeymoon phase
Oftentimes with acute illness, there is an increase in cortisol, which causes an elevation in blood glucose
Patients with an acute illness may require higher insulin doses
Acute illness
1st generation of sulfonylureas
“-amide”
Tolbutamide, chlorpropamide, tolazamide
2nd generation sulfonylureas
“-ride/-zide”
Glyburide, glipizide, glimepiride
both gens are equally effective in lowering blood glucose
sulfonylureas
2nd generation agents are prescribed more often ; they are more potent and have fewer adverse effects and drug interactions
sulfonylureas
Indications of sulfonylureas
Approved for the management of adults with Type 2 DM since they require functional pancreatic beta cells to produce their effect on blood glucose;
NOT FOR TYPE 1 DM
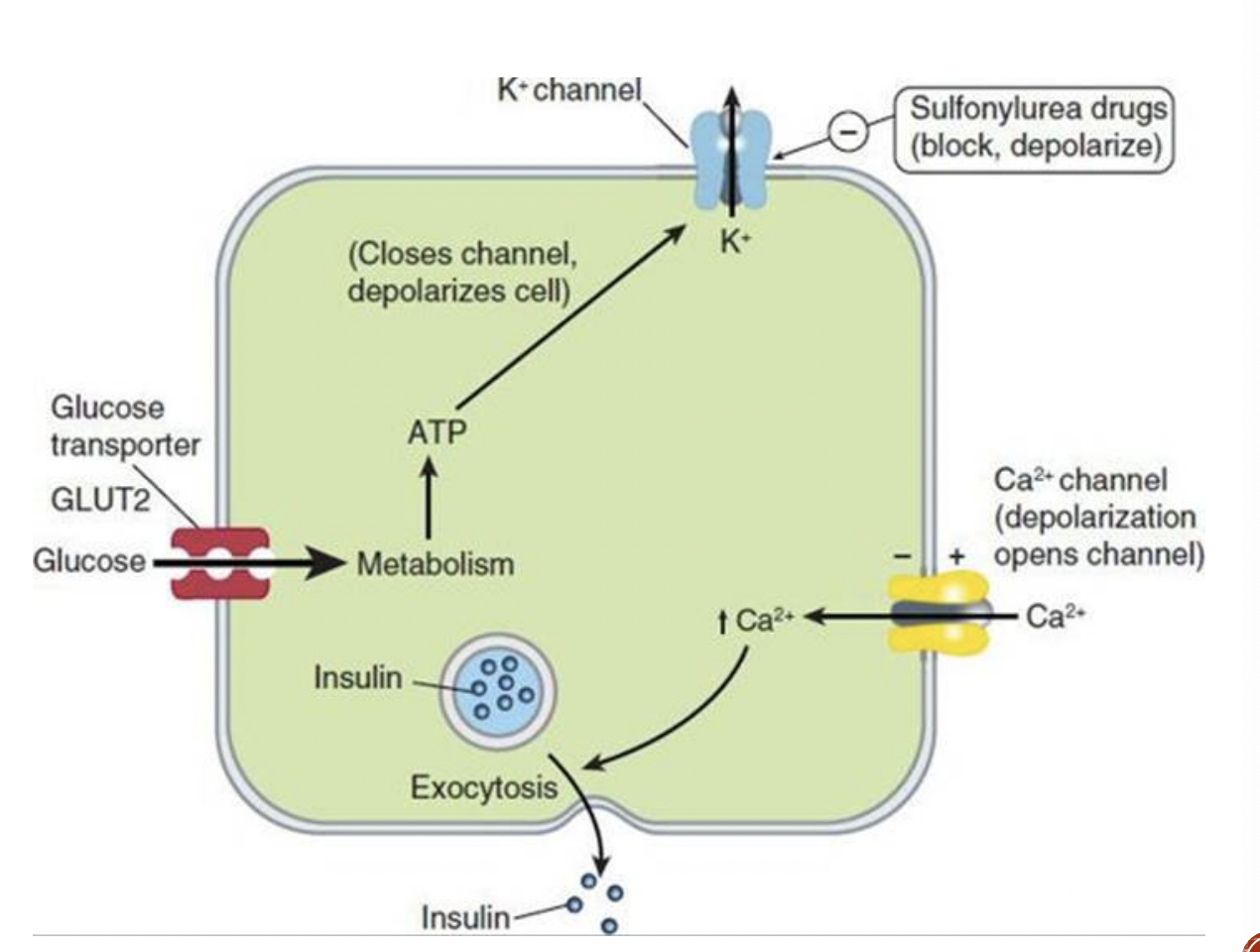
MOA:
These agents are oral insulin secretagogues ; they cause insulin release from pancreatic beta cells
They bind to the SUR1 (sulfonylurea receptor), and block ATP- sensitive K+ channels resulting in depolarization. The voltage gated Ca2+ channels open, resulting in Ca2+ influx and triggering the insulin release
Long term use also reduces serum glucagon, which may contribute to hypoglycemic effects
sulfonylureas MOA
AEs of sulfonylureas
hypoglycemia, weight gain
Precautions of sulfonylureas
caution must be used in patients with hepatic or renal dysfunction ; caution must also be used in patients with a sulfa allergy
Meglitinides
“-linide”
Repaglinide, Nateglinide
MOA: They are oral insulin secretagogues ; Have similar action to sulfonylureas, but they bind to distinct regions on the SUR1 molecule
Meglitinides (Repaglinide, Nateglinide)
indications of Meglitinides (Repaglinide, Nateglinide)
Approved for Type 2 DM
Since they have fast onset and short duration of action, they are recommended in patients with irregular meal schedules and in patients who develop late postprandial hypoglycemia when taking a sulfonylurea
They are used instead of sulfonylureas in patients with a history of sulfa allergy
Adverse effects of Meglitinides (Repaglinide, Nateglinide)
hypoglycemia
what Meglitinides has an AE of weight gain?
Repaglinide
Metformin is what class of drug?
biguanides
Indication of Biguanides
Indication: Type 2 DM
MOA:
It reduces the hepatic glucose production and intestinal absorption of glucose; it does not alter insulin secretion. These effects are believed to be due to an increase in the activity of AMP kinase, a key intracellular regulator of energy homeostasis.
Also increases peripheral insulin sensitivity
Its glucose lowering action does not depend on functional pancreatic beta cells
Biguanides MOA
Does metformin alter insulin secretion?
NO.
It reduces the hepatic glucose production and intestinal absorption of glucose: Biguanides
Advantages of biguanides: metformin
rarely causes hypoglycemia and weight gain
Adverse effects of Biguanides
GI distress, has potential to cause lactic acidosis (characterized by nonspecific symptoms: NV, abdominal pain, lethargy, hyperventilation, and hypotension)
Contraindications of Metformin/Biguanides
Due to an increased risk of lactic acidosis, metformin should not be used in patients with CHF, renal impairment, or who are seriously ill
Metformin should be temporarily discontinued before iodinated contrast, due to the potential for acute kidney injury and increased risk for lactic acidosis
(picture reference of what iodinated contrast means)

Thiazolidinediones
“-litazone”
Pioglitazone, Rosiglitazone
Indications: Type 2 DM
Pioglitazone, Rosiglitazone
MOA:
These agents are insulin sensitizers; they act to decrease insulin resistance
They bind to a specific intracellular receptor, PPAR-y (peroxisome proliferator-activated receptor-gamma), a member of the nuclear-receptor family
They predominantly affect liver, skeletal muscle, and adipose tissue
In the liver, these agents decrease glucose output and insulin levels
In muscle, these agents increase glucose uptake
In adipose tissue, these drugs increase glucose uptake and decrease fatty acid release and may increase the release of hormones such as adiponectin and resistin
The actions of these drugs require the presence of insulin
Can reduce plasma glucose and TG
Thiazolidinediones MOA
Thiazolidinediones indication
Type 2 DM
Adverse Effects of Thiazolidinediones
edema, weight gain
Precautions of Thiazolidinediones
cause an increased risk for fractures and bladder cancer, can cause or exacerbate CHF
Contraindications of Thiazolidinediones
heart failure, liver disease
A-glucosidase inhibitors
Acarbose, Miglitol
MOA:
Act as competitive, reversible inhibitors of pancreatic a- amylase and intestinal a-glucosidase enzymes; they act in the lumen of the intestine
Inhibition of a-glucosidase prolongs the digestion of carbohydrates and reduces peak plasma glucose levels
A-glucosidase inhibitors (Acarbose, Miglitol) MOA
Indications of A-glucosidase inhibitors (Acarbose, Miglitol)
Type 2 DM ; helpful in reducing postprandial glucose
Adverse effects of A-glucosidase inhibitors (Acarbose, Miglitol)
GI distress and flatulence
CI of A-glucosidase inhibitors (Acarbose, Miglitol)
intestinal diseases such as intestinal obstruction & inflammatory bowel disease (IBD)
Glucagon-like Peptide-1 agonists
“-natide, -glutide”
Exenatide, liraglutide, lixisenatide, albiglutide, dulaglutide
Indication of GLP-1 agonists
Type 2 DM ; also cause weight loss
GLP-1 agonist that is tx for T2DM and causes weight loss
Liraglutide
Adverse effect of GLP-1 agonists
GI distress
Precautions of GLP-1 agonists
increased risk for acute pancreatitis and thyroid tumors
Analogs of the hormone incretin (GLP-1)
Increase glucose-dependent insulin secretion; decrease inappropriate glucagon secretion, slow gastric emptying, decrease food intake, and promote B-cell proliferation
GLP-1 agonists MOA
“-liptin”
Sitagliptin, saxagliptin, linagliptin
DPP-4 inhibitors: Dipeptidyl peptidase 4 inhibitors
Indications:
Type 2 DM
DPP-4 inhibitors
AEs of DPP-4 inhibitors
rhinitis and URTI ; may cause pancreatitis
MOA:
DPP-4 is responsible for the proteolysis of incretins, including GLP-1 and glucose-dependent insulinotropic peptide
These agents inhibit DPP-4 to increase active incretins. This leads to an increase in insulin synthesis and release and suppresses glucagon production in a glucose-dependent manner.
DPP-4 inhibitors MOA
amylin analogs
Pramlintide
Amylin analogs: Pramlintide indication
used in combination with insulin for Type2 DM
Amylin analogs: Pramlintide AEs
nausea, hypoglycemia, gastroparesis
MOA:
Amylin is a polypetide stored and secreted by beta cells of the pancreas ; it is cosecreted with insulin to reduce blood sugar. Concentrations are abnormally low in patients with DM.
Pramlintide can reduce postprandial glucose through prolongation of gastric emptying, reduction of prostprandial glucagon secretion, and reduction of caloric intake through centrally mediated appetite suppression.
Causes weight loss and reduces postprandial glucose levels
Amylin analogs: Pramlintide MOA
SGLT-2 inhibitors
Canagliflozin, empagliflozin, dapagliflozin
SGLT-2 inhibitors
Indications: Type 2 DM
advantages of SGLT-2: Canagliflozin, empagliflozin, dapagliflozin
weight loss and a modest decrease in BP
Adverse effects of SGLT-2 inhibitors
include genitourinary infections and increased serum potassium
CI of SGLT-2 inhibitors
severe renal impairment
MOA:
SGLT2 is the main site of filtered glucose reabsorption
These agents inhibit SGLT2 in the proximal renal tubules; this results in reduced reabsorption of filtered glucose from the tubular lumen and lowers the renal threshold for glucose (RTG)
Reduction of filtered glucose reabsorption and lowering of RTG results in increased urinary excretion of glucose, thereby reducing plasma glucose concentrations.
SGLT2 inhibitors MOA
hyperglycemic agents
glucagon
diazoxide
MOA:
Stimulates adenylate cyclase to produce increased cAMP
It increases blood glucose by stimulating glycogenolysis and gluconeogenesis in the liver
In general, its actions oppose the actions of insulin
Large doses produce marked relaxation of the smooth muscle in the stomach, intestines and colon.
glucagon
MOA: this agent opens ATP-dependent potassium channels on pancreatic beta cells, resulting in inhibition of insulin release.
diazoxide
indication of diazoxide
hyperinsulinemic hypoglycemia
diazoxide AEs
sodium retention, GI distress, and changes in circulating white blood cells