Biochem - 9.4 notes (book + lect)
1/366
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
367 Terms
Q: What two factors control enzyme activity in metabolic pathways?
A: Substrate availability and levels of catalytic activity determine enzyme activity and therefore control metabolic flux.
Q: How are reactions with ΔG values near zero different from those with large negative ΔG values?
ΔG ≈ 0: reactions are reversible, direction depends mainly on substrate availability.
Highly negative ΔG: reactions are irreversible and under enzymatic control, forming key regulation points.
Q: Which three enzymes in glycolysis are functionally irreversible and regulated?
Hexokinase (Reaction 1)
Phosphofructokinase-1 (Reaction 3)
Pyruvate kinase (Reaction 10)
Q: What defines a rate-limiting reaction in metabolism?
A: It is the slowest reaction in the pathway, often irreversible, acting as a regulated “valve” that adjusts flux in response to cellular conditions.
Q: How are glycolysis and gluconeogenesis linked in terms of enzyme control?
A: Both share six reversible enzymes but have three (glycolysis) or four (gluconeogenesis) pathway-specific enzymes for the irreversible steps, controlling direction and metabolic flux.
Q: Compare the energy balance of glycolysis and gluconeogenesis.
Glycolysis: catabolic, yields +2 ATP per glucose.
Gluconeogenesis: anabolic, consumes 4 ATP + 2 GTP.
Both are highly favorable overall (ΔG°′ = –85.0 kJ/mol and –47.6 kJ/mol respectively).
Q: What are the three main ways substrate availability and enzyme activity control glycolytic flux?
Glucokinase/hexokinase regulation (substrate capture and sensing).
Allosteric control of PFK-1 (energy charge-dependent).
Metabolite inflow/outflow (substrate concentration effects
Q: What is glucokinase’s unique role in liver and pancreatic cells?
A: It acts as a molecular sensor of high blood glucose, catalyzing glucose → glucose-6-P only when glucose levels are elevated.
Q: How are glycolytic enzymes also regulated in the long term?
A: Transcriptional regulation in response to high-carbohydrate diets upregulates genes such as PFK-1, glyceraldehyde-3-P dehydrogenase, and pyruvate kinase.
Q: How many hexokinase isoenzymes exist in humans, and what is their general function?
A: Four (I–IV); all catalyze glucose → glucose-6-P using ATP.
Q: How do hexokinase I and glucokinase (IV) differ?
Property | Hexokinase I | Glucokinase (IV) |
|---|---|---|
Km for glucose | ≈ 0.1 mM (high affinity) | ≈ 10 mM (low affinity) |
Tissue distribution | All tissues | Liver & pancreatic cells |
Substrate specificity | Broad (hexoses) | Specific for glucose |
Product inhibition | Inhibited by G-6-P | Not inhibited by G-6-P |
Physiologic role | Basal glucose phosphorylation | Glucose sensor for blood glucose control |
Q: Why does glucokinase only act when blood glucose > 5 mM?
A: Because of its high Km, it becomes active only at post-meal (carbohydrate-rich) glucose concentrations.
Q: How does glucokinase activity help regulate blood glucose?
A: By converting excess glucose to glucose-6-P, which is then turned into glucose-1-P (via phosphoglucomutase) for glycogen synthesis, trapping glucose in liver cells.
Q: How does glucokinase function as a glucose sensor in pancreatic β cells?
A: Elevated glucose → increased GLUT2 transport → glucokinase activation → ↑ ATP via glycolysis → closure of ATP-sensitive K⁺ channels → depolarization → opening of voltage-gated Ca²⁺ channels → insulin secretion.
Q: Why is PFK-1 considered the key regulatory enzyme of glycolysis?
A: It controls flux through the pathway, integrating signals of cellular energy status.
Q: Which metabolites act as allosteric activators and inhibitors of PFK-1?
Activators: AMP, ADP, and fructose-2,6-bisphosphate (F-2,6-BP).
Inhibitors: ATP (high energy charge) and citrate.
Q: How does cellular energy charge affect PFK-1 conformation?
High ATP: favors inactive T-state → low affinity for fructose-6-P.
High AMP/ADP: favors active R-state → enhanced fructose-6-P binding.
Q: What is the role of fructose-2,6-bisphosphate (F-2,6-BP)?
A: It is a potent feed-forward activator that increases PFK-1 activity when fructose-6-P accumulates.
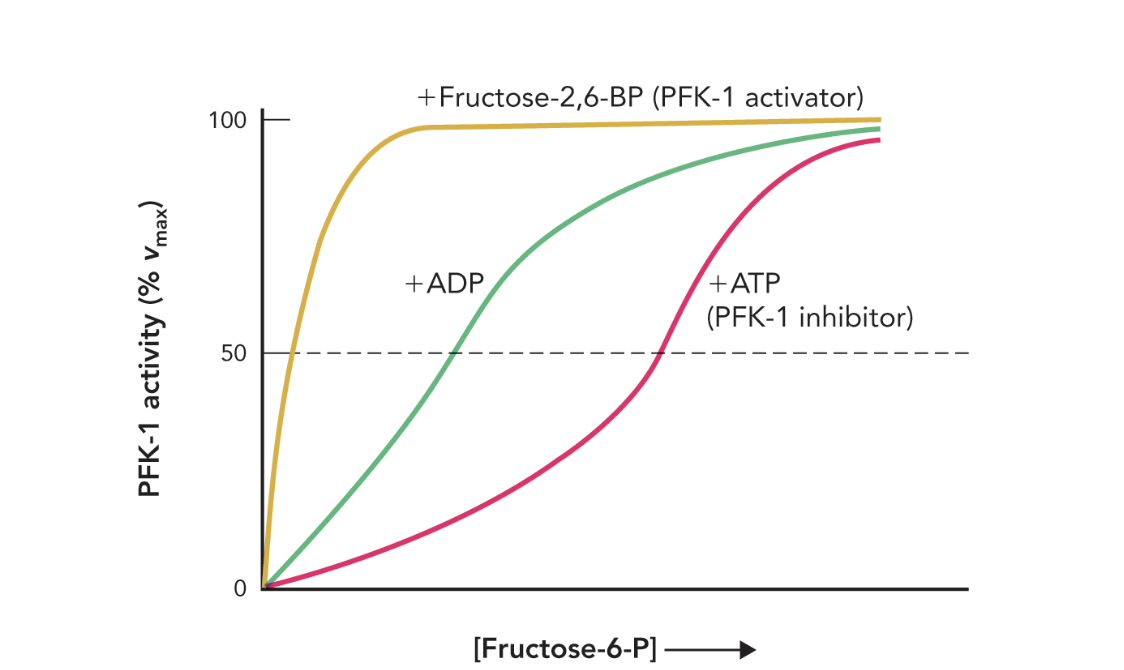
Q: How does Figure 9.42 illustrate PFK-1 regulation?
A: It shows that high ATP shifts the activity curve right (inhibition), while F-2,6-BP and ADP restore activity by preventing ATP binding to the allosteric site.
Q: How is pyruvate kinase regulated allosterically?
A: Pyruvate kinase’s tetrameric active form is stabilized by fructose-1,6-bisphosphate, linking reactions 3 and 10 in feed-forward regulation—increased F-1,6-BP signals elevated upstream glycolytic flux.
Q: How does ATP affect pyruvate kinase activity?
A: High ATP levels allosterically inhibit pyruvate kinase, decreasing glycolytic flux when cellular energy is sufficient.
Q: What happens when glycolytic flux decreases?
A: Excess glucose accumulates in liver and muscle cells, converted to glycogen for storage of metabolic energy.
Q: What are the main sources of glucose for glycolysis in animals?
A: Glucose derives from dietary carbohydrates and glycogen, the polymeric storage form of glucose.
Q: How do plants and animals process dietary starch?
A: α-Amylase hydrolyzes starch → maltose (a disaccharide), which is then cleaved by maltase → 2 glucose molecules for glycolysis.
Q: How are sucrose and lactose metabolized?
Sucrose → glucose + fructose (via sucrase)
Lactose → glucose + galactose (via lactase)
Q: What enzyme releases glycerol from triglycerides, and where does it go?
A: Lipase enzymes hydrolyze triglycerides, releasing glycerol, which can enter glycolysis.
Q: Why are infants able to digest lactose more efficiently than adults?
A: The lactase gene is highly expressed in infants (for milk digestion) but downregulated in adults.
Q: What causes lactose intolerance symptoms?
A: Undigested lactose is fermented by Lactobacillus bacteria → lactate, H₂, and CH₄, increasing osmotic pressure and water flow into the intestine → diarrhea and gas.
Q: How do fructose, galactose, and glycerol enter glycolysis?
A: Through specific enzymatic reactions converting them to intermediates like glyceraldehyde-3-P and dihydroxyacetone-P (see Figure 9.45).
Q: Describe glycerol’s entry route into glycolysis.
Glycerol kinase: glycerol → glycerol-3-P
Glycerol-3-P dehydrogenase: glycerol-3-P → dihydroxyacetone-P
Q: How is fructose metabolized in muscle vs. liver cells?
Muscle: fructose → fructose-6-P (via hexokinase).
Liver: fructose → fructose-1-P (via fructokinase) → DHAP + glyceraldehyde (via aldolase B).
Q: What causes hereditary fructose intolerance?
A: Deficiency in fructose-1-P aldolase (aldolase B) prevents metabolism of fructose-1-P, which accumulates in the liver.
Q: Why is fructose-1-P accumulation toxic?
A: It sequesters inorganic phosphate (Pi), depleting ATP needed for membrane pumps, causing cell lysis and liver damage.
Q: What are the four enzymatic steps of galactose metabolism?
Galactokinase: galactose → galactose-1-P
Galactose-1-P uridylyltransferase: galactose-1-P + UDP-glucose → UDP-galactose + glucose-1-P
UDP-galactose 4-epimerase: UDP-galactose → UDP-glucose
Phosphoglucomutase: glucose-1-P → glucose-6-P (enters glycolysis)
Q: What causes galactosemia, and what are its symptoms?
A: Defects in galactose-1-P uridylyltransferase cause galactosemia, leading to galactose buildup in blood/urine, liver damage, and developmental delays in infants. Early diagnosis and a galactose-free diet can reverse symptoms.
Q: What anabolic functions do glycolytic intermediates serve?
A: They provide carbon skeletons for biosynthesis, such as:
Glucose-6-P → pentose phosphates for nucleotide synthesis & NADPH production (pentose phosphate pathway).
Glucose-6-P → glucose-1-P → glycogen (storage).
DHAP → glycerol phosphate → triglyceride synthesis.
Q: How do ATP, ADP, and AMP together regulate glycolytic rate?
A: The cell’s energy charge (ratio of ATP:ADP:AMP) coordinates glycolytic enzymes—high ATP suppresses PFK-1 and pyruvate kinase; high AMP/ADP relieve inhibition—ensuring glycolytic flux matches cellular energy needs.
Q: How do short-term and long-term controls of glycolysis differ?
Short-term: allosteric and covalent enzyme regulation (e.g., PFK-1, pyruvate kinase).
Long-term: transcriptional control in response to carbohydrate intake, adjusting enzyme expression (PFK-1, GAPDH, pyruvate kinase).
Q: What does feed-forward regulation of pyruvate kinase accomplish?
A: It synchronizes glycolysis: when PFK-1 produces more F-1,6-BP, it signals pyruvate kinase to increase activity downstream, maintaining pathway continuity and preventing intermediate buildup.
Q: Despite varied carbohydrate sources, where do alternative sugars feed into glycolysis?
A: Most converge at glyceraldehyde-3-phosphate or dihydroxyacetone-phosphate, integrating seamlessly into stage 2 of glycolysis.
Q: How do hereditary enzyme deficiencies highlight glycolysis’s metabolic centrality?
A: Disorders like fructose intolerance (aldolase B deficiency) and galactosemia (uridyltransferase deficiency) show how blocked sugar entry disrupts phosphate balance, ATP generation, and even causes liver cell death—demonstrating glycolysis’s systemic importance.
Q: What controls metabolic flux through opposing pathways like glycolysis and gluconeogenesis?
A: Rate-limiting enzymes that are highly regulated control flux through opposing pathways.
Q: Where do the “bottlenecks” in glycolysis and gluconeogenesis occur?
A: At irreversible steps in the pathway, catalyzed by enzymes with large negative ΔG values.
Q: How are reversible and irreversible reactions represented in metabolic diagrams?
A: Reversible reactions are shown as wide regions (unrestricted flow), while irreversible steps are narrow “bottlenecks.”
Q: Why do glycolysis and gluconeogenesis use different enzymes for certain steps?
A: To bypass irreversible reactions and allow metabolic flow in opposite directions without energy waste.
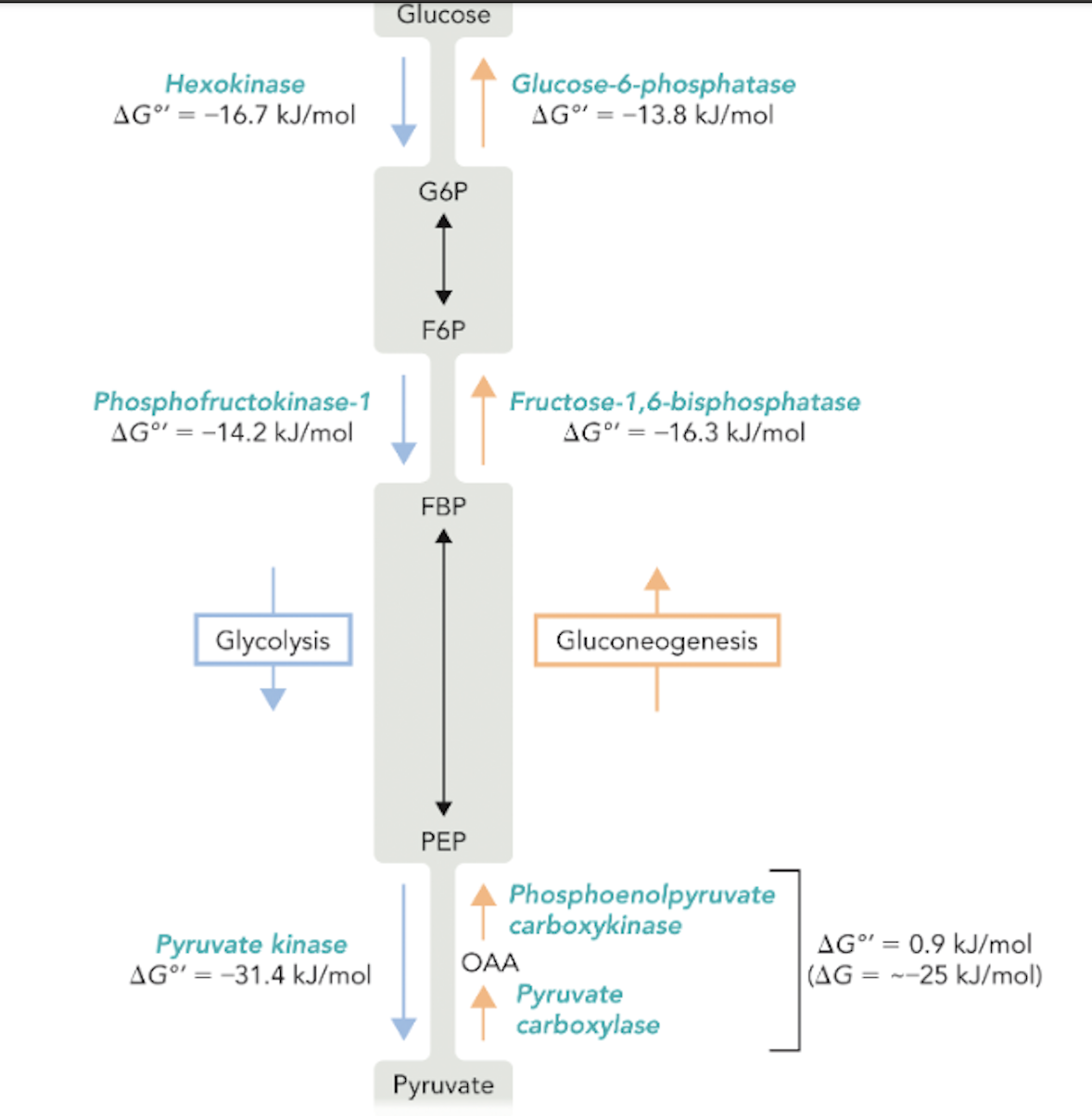
Q: What is the overall ΔG°′ for the combined pyruvate carboxylase + PEPCK reactions in gluconeogenesis?
A: Approximately 0.9 kJ/mol, but the actual ΔG is about −25 kJ/mol because PEP is rapidly metabolized.
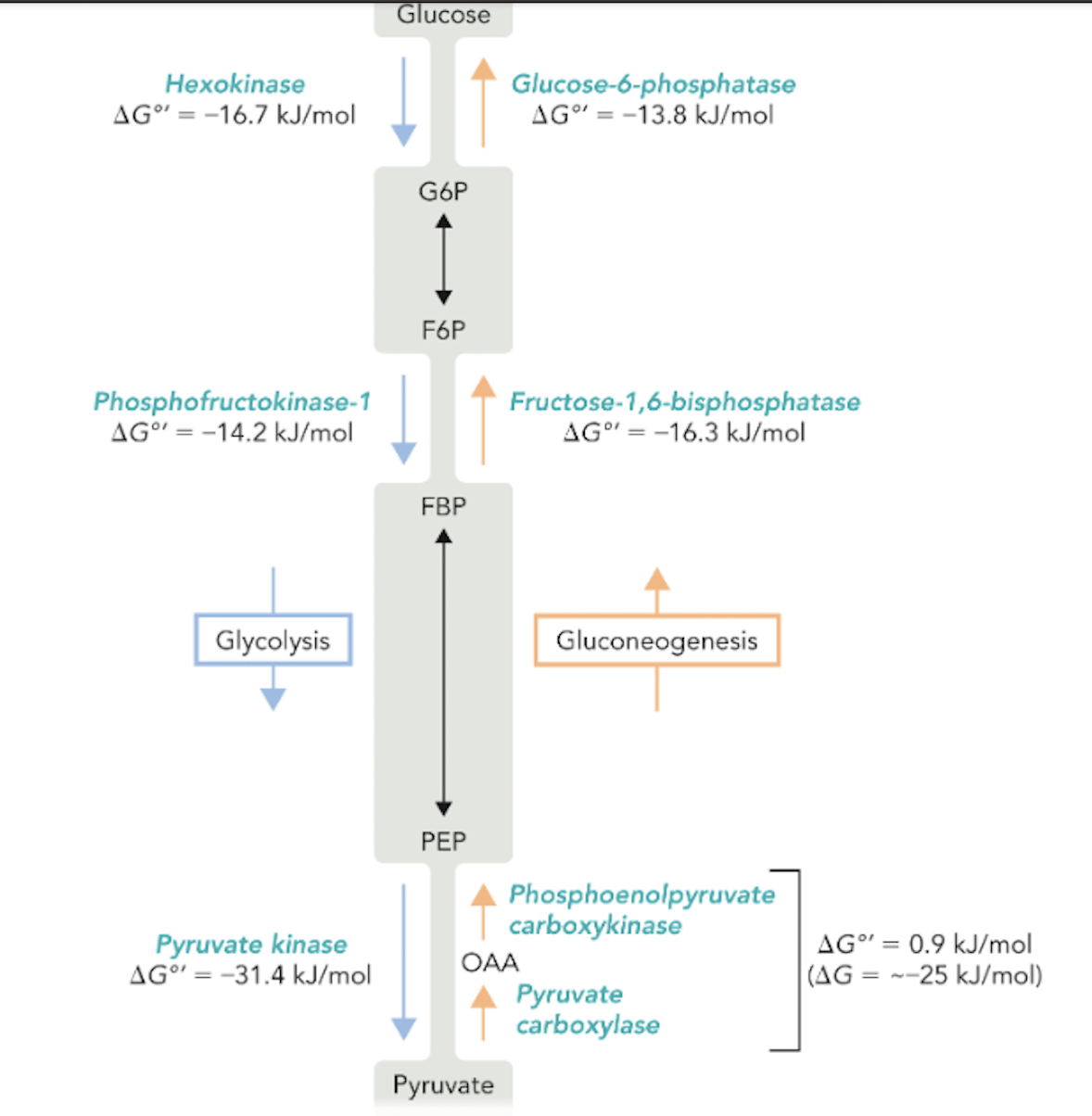
Q: What are the main metabolites shared between glycolysis and gluconeogenesis?
A: G6P (glucose-6-phosphate), F6P (fructose-6-phosphate), FBP (fructose-1,6-bisphosphate), PEP (phosphoenolpyruvate), OAA (oxaloacetate).
Q: Why is the regulation of glycolysis and gluconeogenesis essential?
A: It prevents both pathways from operating simultaneously, which would waste ATP and disrupt energy balance.
Q: Which enzymes catalyze the three irreversible steps of glycolysis?
A: Hexokinase, phosphofructokinase-1, and pyruvate kinase.
Q: Which enzymes bypass these steps in gluconeogenesis?
A: Glucose-6-phosphatase, fructose-1,6-bisphosphatase, pyruvate carboxylase, and PEP carboxykinase.
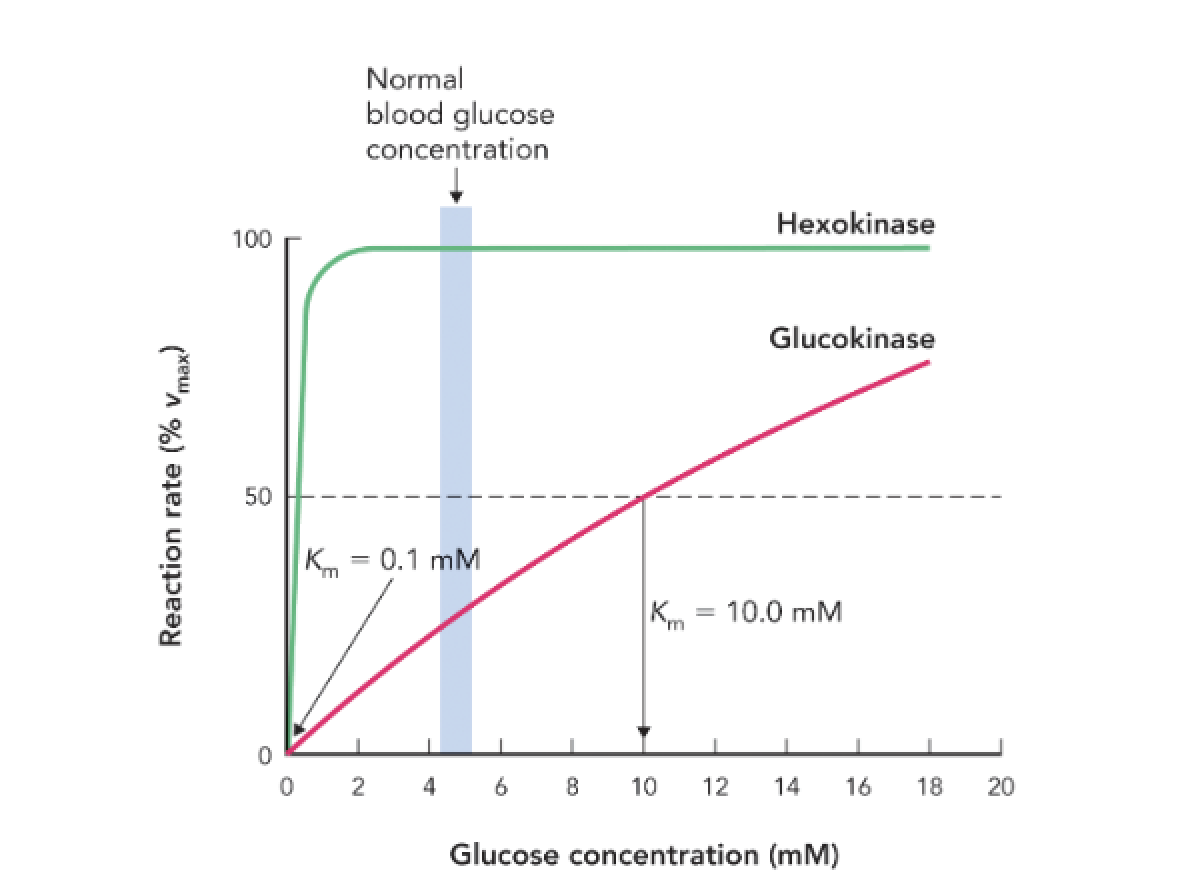
Q: What reaction do both hexokinase and glucokinase catalyze?
A: The phosphorylation of glucose to glucose-6-phosphate (G6P) using ATP.
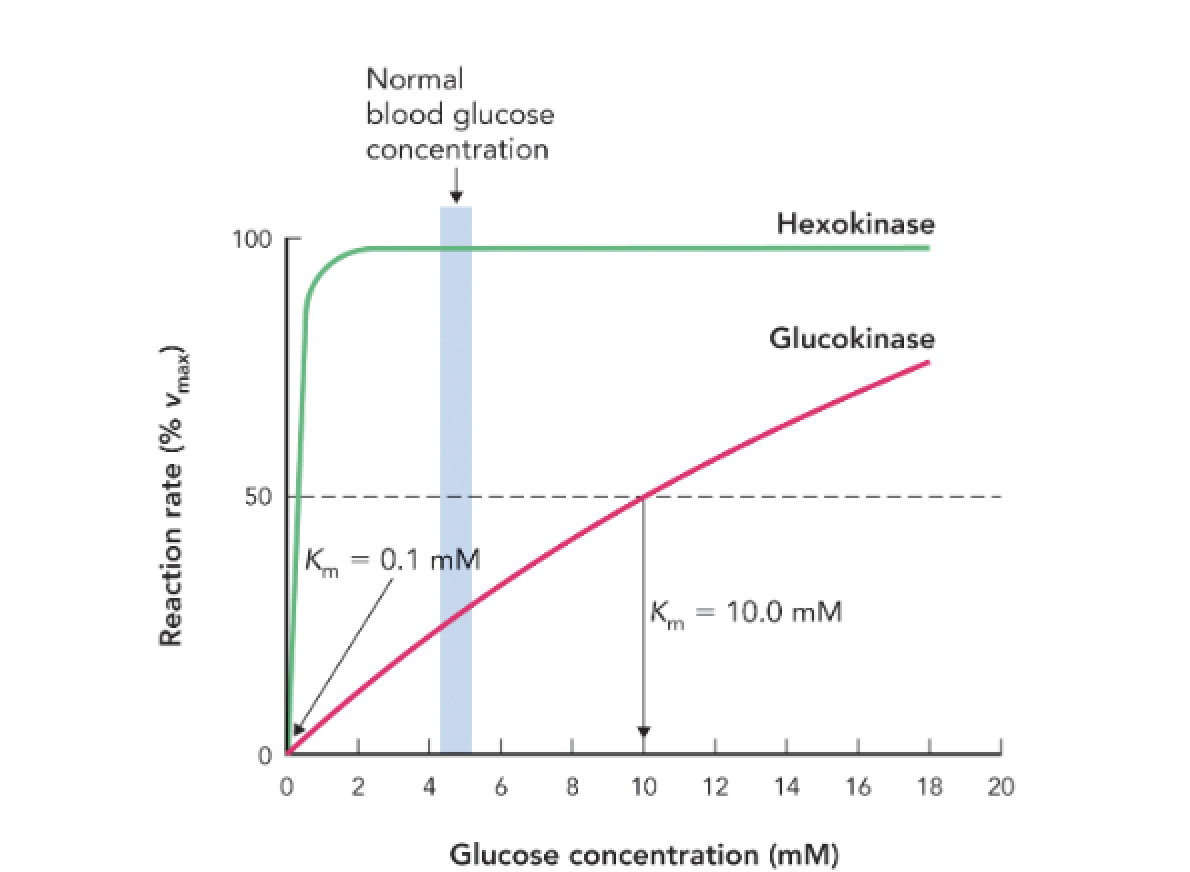
Q: What is the Km value for hexokinase and what does it indicate?
A: Km = 0.1 mM, indicating high affinity for glucose; it is active even at low glucose concentrations.
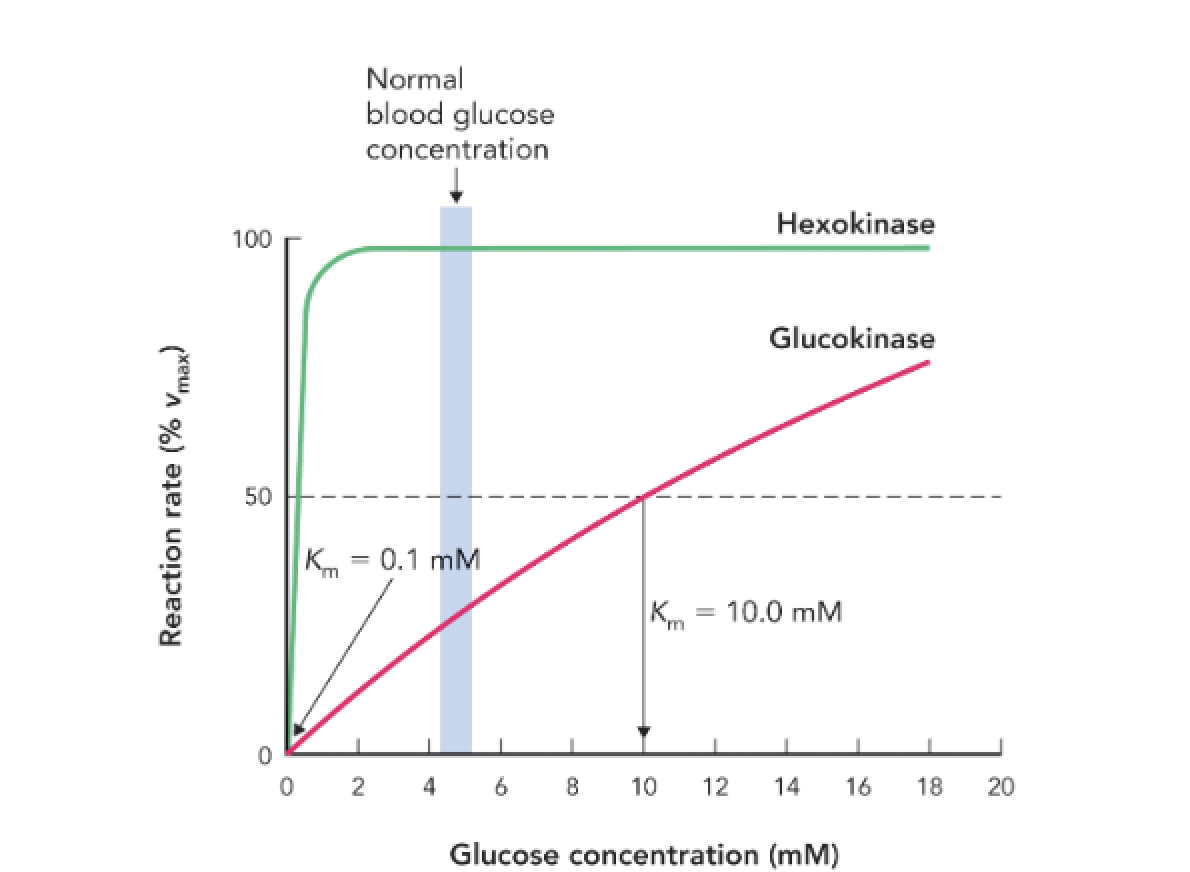
Q: What is the Km value for glucokinase and what does it indicate?
A: Km = 10 mM, indicating low affinity for glucose; it becomes active only when glucose is abundant.
Q: How do the kinetic differences between hexokinase and glucokinase regulate glucose metabolism?
A: Hexokinase ensures glucose utilization in most tissues at all times, while glucokinase in the liver and pancreas becomes active only when glucose levels are high, helping regulate storage and insulin secretion.
Q: At normal blood glucose levels (~5 mM), which enzyme is more active?
A: Hexokinase is near full activity; glucokinase is only moderately active.
Q: What is the physiological role of glucokinase in the liver?
A: To sequester glucose by phosphorylating it to G6P when glucose is abundant, promoting glycogen synthesis and maintaining blood glucose homeostasis.
Q: What key regulatory function does glucokinase serve in pancreatic β cells?
A: It acts as a glucose sensor, triggering insulin release when blood glucose levels rise.
Q: What role does glucokinase play in pancreatic β cells?
A: It acts as a glucose sensor, initiating insulin secretion when blood glucose levels rise.
Q: How does glucose enter pancreatic β cells?
A: Through the GLUT2 transporter, which facilitates glucose entry when blood glucose levels are high.
Q: What reaction does glucokinase catalyze in β cells?
A: The phosphorylation of glucose to glucose-6-phosphate, the first step of glycolysis.
Q: How does ATP production influence K⁺ channels in β cells?
A: Increased ATP levels inhibit ATP-sensitive K⁺ channels, preventing K⁺ efflux and leading to membrane depolarization.
Q: What happens after membrane depolarization?
A: Voltage-gated Ca²⁺ channels open, allowing Ca²⁺ influx into the cell.
Q: How does Ca²⁺ trigger insulin release?
A: It stimulates fusion of insulin vesicles with the plasma membrane, releasing insulin into the blood.
Q: Why is the negative charge inside the β cell important?
A: The resting negative potential enables depolarization to act as a signal for Ca²⁺ entry and insulin secretion.
Q: What ensures insulin is only released when blood glucose is high?
A: The high Km of glucokinase and the ATP-dependent signaling cascade that only activates under high glucose conditions.
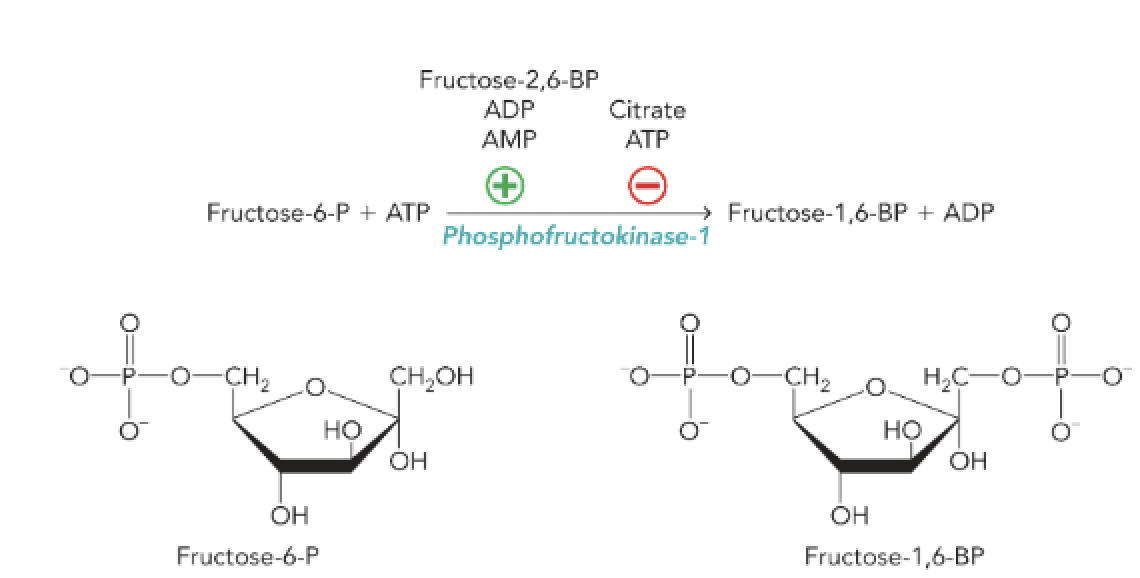
Q: What is the main function of phosphofructokinase-1 (PFK-1)?
A: PFK-1 catalyzes the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate using ATP, controlling a key regulatory step in glycolysis.
Q: Which metabolites act as allosteric activators of PFK-1?
A: AMP, ADP, and fructose-2,6-bisphosphate (F-2,6-BP).
Q: Which metabolites inhibit PFK-1 allosterically?
A: ATP and citrate.
Q: What does high ATP or citrate concentration signal to PFK-1?
A: That the cell has high energy charge or abundant biosynthetic intermediates, leading to inhibition of PFK-1 and a slowdown in glycolysis.
Q: What does high AMP or ADP concentration signal to PFK-1?
A: That the cell has low energy charge, leading to activation of PFK-1 and increased glycolytic flux to generate more ATP.
Q: How does fructose-2,6-bisphosphate regulate glycolysis?
A: It is a potent allosteric activator of PFK-1 that stimulates glycolysis even when ATP levels are moderately high.
Q: Where are the allosteric binding sites for AMP, ADP, and ATP located on PFK-1?
A: In close proximity to the substrate-binding sites, allowing allosteric effectors to change enzyme conformation and activity.
Q: What reaction in glycolysis is controlled by PFK-1?
A: Reaction 3 – the conversion of fructose-6-phosphate + ATP → fructose-1,6-bisphosphate + ADP.
Q: How does citrate function as an inhibitor of PFK-1?
A: It signals that the citric acid cycle is saturated, preventing excessive glycolytic flux and accumulation of intermediates.
Q: What type of enzyme structure does PFK-1 have?
A: PFK-1 is a homotetramer, composed of four identical subunits.
Q: Where are the allosteric binding sites of PFK-1 located?
A: At the interface between subunits, allowing allosteric effectors to influence catalytic activity by changing subunit orientation.
Q: What effectors stabilize the inactive conformation of PFK-1?
A: ATP and citrate — they bind to regulatory sites and inhibit the enzyme.
Q: What effectors stabilize the active conformation of PFK-1?
A: ADP (and AMP) — they bind to regulatory sites and activate the enzyme.
Q: How do allosteric effectors alter PFK-1 activity?
A: They change the relative positions of subunits, altering the enzyme’s conformation and thus its catalytic efficiency.
Q: What is the result of ATP or citrate binding to PFK-1?
A: The enzyme adopts an inactive conformation, reducing glycolytic flux.
Q: What is the result of ADP binding to PFK-1?
A: The enzyme adopts an active conformation, promoting glycolytic flux.
Q: What are the PDB structures associated with inactive and active PFK-1 conformations?
Inactive: 6PFK (ATP or citrate bound)
Active: 4PFK (ADP bound)
Q: What is the overall significance of PFK-1 allosteric regulation?
A: It allows fine-tuned control of glycolysis based on cellular energy levels, making PFK-1 the pathway’s key regulatory enzyme.
Q: What are the two conformational states of phosphofructokinase-1 (PFK-1)?
A: The T state (tense/inactive) and the R state (relaxed/active).
Q: What stabilizes the T (inactive) state of PFK-1?
A: ATP, when cellular energy charge is high.
Q: What stabilizes the R (active) state of PFK-1?
A: ADP (or AMP), when cellular energy charge is low.
Q: What happens to fructose-6-phosphate (F6P) binding in the T state?
A: F6P binding is weakened, reducing enzyme activity and glycolytic flux.
Q: What happens to F6P binding in the R state?
A: F6P binding is enhanced, leading to product formation and active glycolysis.
Q: How does ATP function as both a substrate and an inhibitor of PFK-1?
A: ATP is a substrate at the catalytic site but an inhibitor when bound to the regulatory allosteric site.
Q: How does the ATP/ADP ratio affect glycolysis?
A:
High ATP/low ADP → Inhibits glycolysis (T state favored).
Low ATP/high ADP → Activates glycolysis (R state favored).
Q: Why is the R ↔ T transition important for metabolism?
A: It allows fine-tuned feedback control, ensuring glycolysis meets the cell’s energy demands efficiently.
Q: Why is the enzyme drawn as a dimer in this figure?
A: For simplicity; PFK-1 actually functions as a tetramer in vivo.
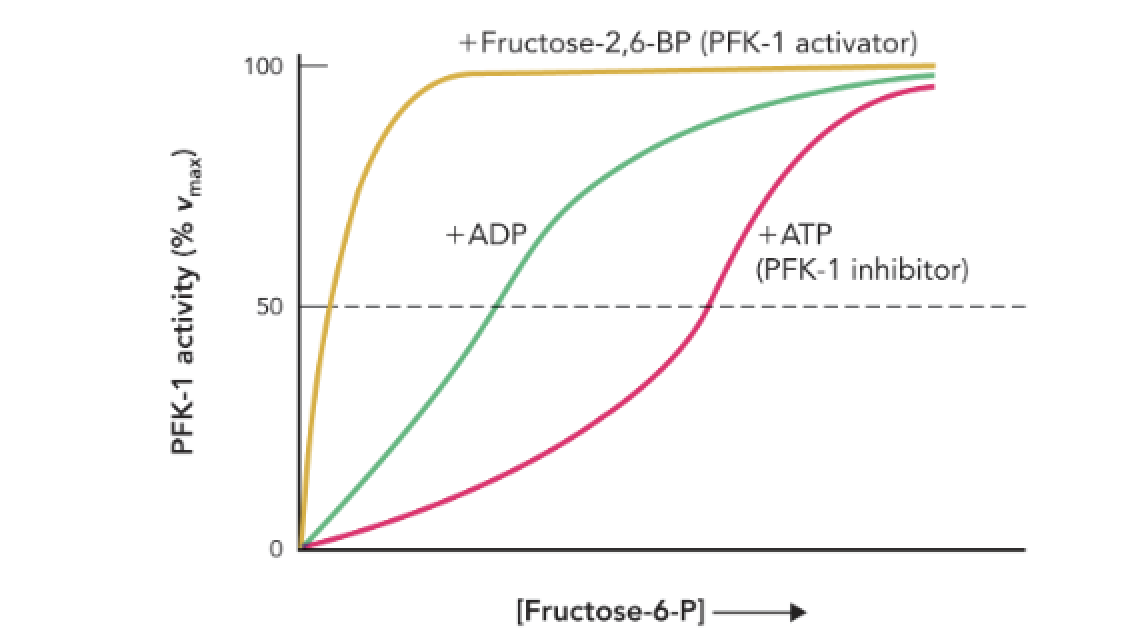
Q: What does Figure 9.42 show about PFK-1 activity?
A: It shows how allosteric regulators (ATP, ADP, and fructose-2,6-bisphosphate) affect PFK-1 enzyme activity as [fructose-6-phosphate] increases.
Q: How does ATP affect PFK-1 activity?
A: ATP acts as an allosteric inhibitor, decreasing the enzyme’s affinity for F6P and slowing glycolysis.
Q: How does ADP affect PFK-1 activity?
A: ADP acts as an allosteric activator, increasing the enzyme’s affinity for F6P and stimulating glycolysis.
Q: Which molecule is the strongest activator of PFK-1?
A: Fructose-2,6-bisphosphate (F-2,6-BP) — it causes maximal enzyme activation even at low [F6P].
Q: What does the rightward shift in the ATP curve indicate?
A: It indicates inhibition — the enzyme requires higher [F6P] to reach the same activity level.
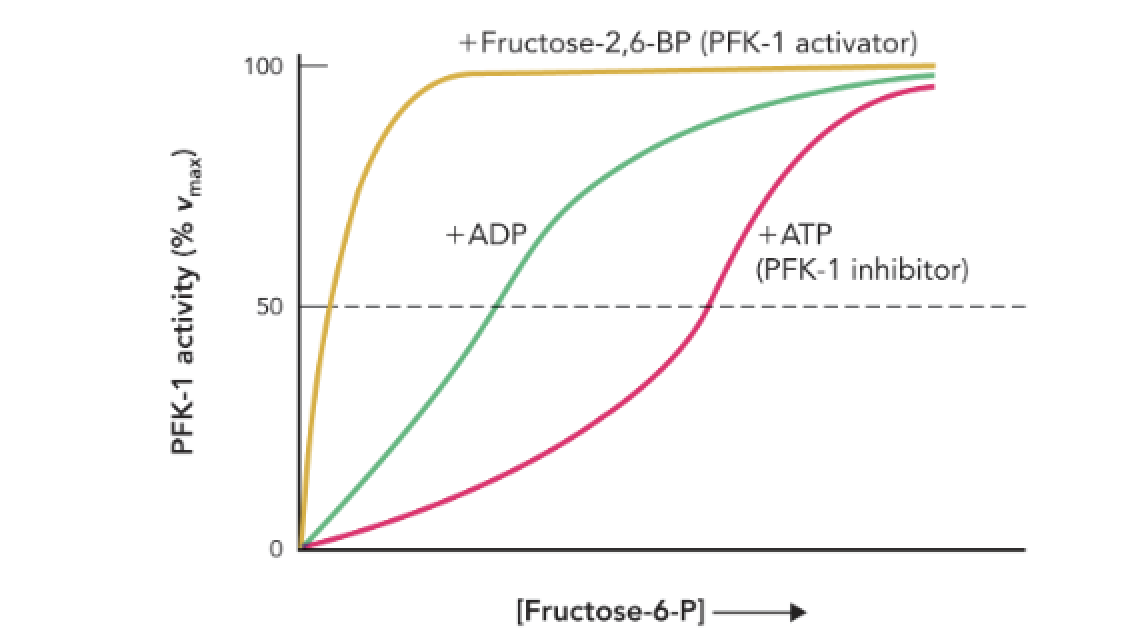
Q: What does the leftward shift in the ADP and F-2,6-BP curves indicate?
A: It indicates activation — the enzyme achieves high activity at lower [F6P].
Q: Why is F-2,6-BP important in glycolytic regulation?
A: It links hormonal signals (insulin/glucagon) to glycolytic control, strongly activating PFK-1 when glucose availability is high.
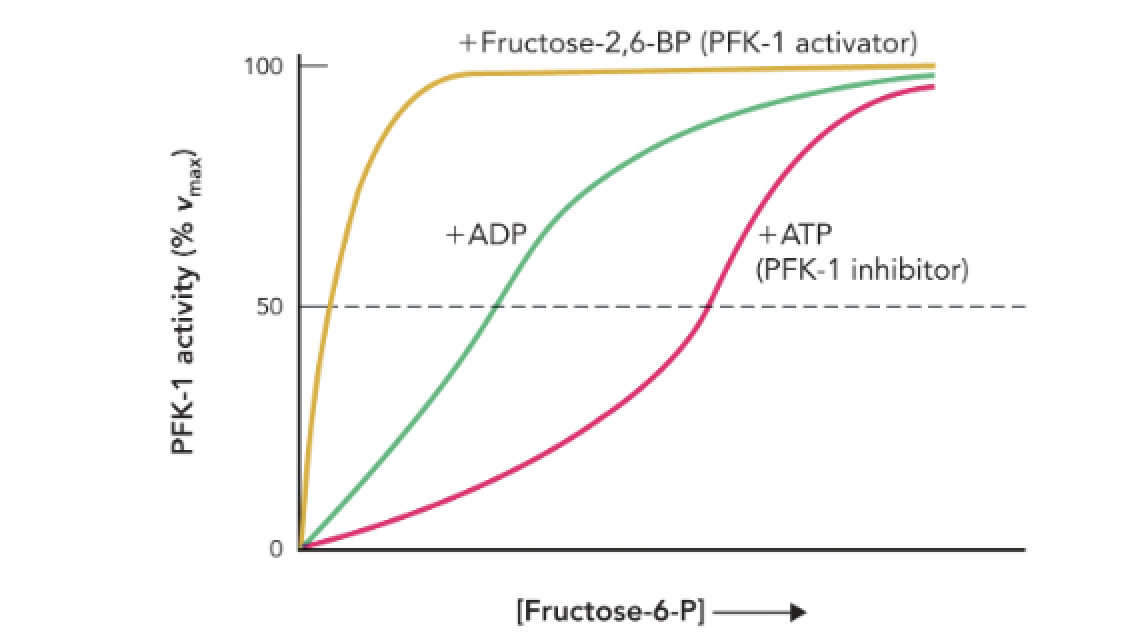
Q: What does the shape of each curve represent?
A: The relative effect of each allosteric regulator on enzyme kinetics at its maximal concentration.