24-25. retroviridae I & II
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
where did human immunodeficiency viruses come from?
zoonosis → multiple simian immunodeficiency viruses that jumped into human hosts
what type of genome do retroviruses have?
RNA
are retroviruses naked or enveloped?
enveloped
envelope proteins often heavily glycosylated → masking from antibodies
what types of cells do retroviruses frequently target?
lymphocytes and/or macrophages → systemic spread, proliferation of infected cells
unique/notable properties of retroviruses
reverse transcriptase: RNA → DNA
error-prone → high mutation rate; rapid evolution
diploid particles (2 RNA copies) → facilitates within-host recombination
integration of DNA provirus into host cell’s genome
enables long-lived, persistent, latent infections
cancer: insertional mutagenesis; disruption of normal gene expression
how can oncogenic retroviruses cause cancer?
through recombination, retroviruses can acquire cellular oncogenes → these mutate through viral passage, becoming viral oncogenes (v-onc)
viral oncogene-encoding viruses transduce the oncogene, rapidly causing cancer
require “helper viruses”; no longer able to recompilation on their own
viruses can insert next to a cellular oncogene and upregulate its expression, causing cancer more slowly
(complex retroviruses may have other oncogenic mechanisms)
feline lymphoma & FeLV infection
lymphoma usually manifests as multicentric or thymic T cell tumors, frequently in cats < 3 y/o
multicentric, mediastinal: associated with FeLV; decreasing incidence
GI lymphomas: B cell tumors; more common currently; generally in older cats
tumors develop slowly after FeLV infection → upregulation of cellular oncogene c-myc
FeLV variants
only FeLV-A is replication-competent and transmissible among animals
other forms arise in infected cats through mutation — recombination between FeLV & endogenous retroviruses or cellular proto-oncogenes
non-A viruses occur only as co-infections, with FeLV-A as their helper virus
these variants are not replication-competent
multiple variants have been described in association with specific disease complexes
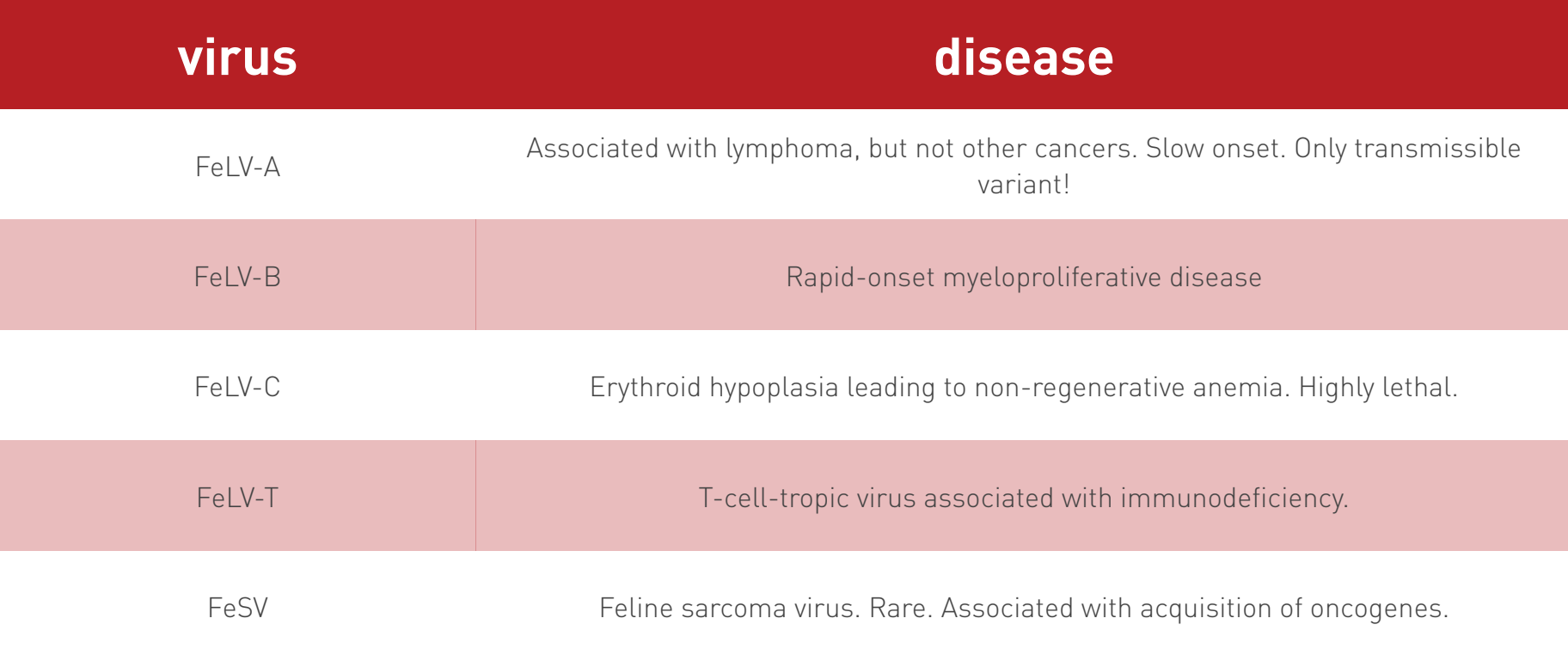
FeLV progressive vs. regressive infection
progressive infection (high-positive)
weaker immune response → higher levels of FeLV provirus & antigen
persistent high viremia & eventual disease
regressive infection (low-positive)
stronger immune response → lower levels of FeLV provirus & antigen
(apparent) clearance of antigenemia; potential for longer survival
primary & secondary FeLV infection
primary: initial infection; characterized by infection of lymphoid tissue
secondary: infection of bone marrow sets up persistent viremia and progressive infection
abortive FeLV infection
no viral antigen or DNA detected, but antibodies present
FeLV clinical signs
nonspecific symptoms
fever of unknown origin
lymphadenopathy
immunosuppression
wasting
abortion
anemia
cattery history
FeLV diagnosis
serology most common → ELISA and IFA tests for FeLV antigen
level 1: ELISA tests detect cell-free virus
screening test; serum
level 2: PCR for proviral DNA and RT-PCR for genomic RNA; or IFA (hardy test) to detect cell-associated virus
used to confirm positive ELISA
PCR commercially available
whole blood may be best sample type
FeLV transmission
secreted in saliva, blood, urine, feces
primarily horizontal transmission → prolonged friendly contact
dose required for oronasal transmission is likely high
can also occur in utero or perinatally
FeLV control
test and remove; isolate infected animals
test prior to vaccination, breeding, or co-housing; test all sick cats
do not decide to euthanize on the basis of (+) ELISA alone
vaccinate
may prevent clinical disease but not infection
highly recommended for all kittens
2 dose initial series, followed by booster and assessment for exposure risk
what neoplasm is associated with bovine leukosis virus? what organs does it typically affect?
lymphosarcomas
organs affected:
heart (right atrium)
uterus
nodules in central and peripheral lymph nodes
abomasum
other sites, causing additional tissue-specific signs
BLV & persistent lymphocytosis
PL develops after a latent period lasting from months to several years
detectable in blood samples, but otherwise not clinically apparent
distinct from leukemia → normal lymphocytes; could become leukemia
increases animal’s risk of developing leukemia by 2x
BLV diagnosis
routinely diagnosed by presence of antiviral antibody (after 6 months of age)
PCR tests for provirus also available
BLV pathogenesis
mechanism not completely defined, but involves viral Tax protein, a transcriptional transactivator
implicated in oncogenesis
inhibition of apoptosis?
may promote viral “sneakiness” by downregulating viral gene expression
BLV clinical signs
tumors typically take several years to develop
once animals develop symptomatic disease, frequently suffer weight loss and milk production is greatly reduced
lymphosarcoma is rapidly progressive → wasting, death within 4 months of diagnosis
BLV transmission
iatrogenic transmission most common (exposure to infected cells)
BLV is strongly cell-associated, so not easily transmitted among animals
vertical transmission ineffecient
horizontal transmission among adult cattle
calves can become infected after birth (exposure to exudate & placenta from cows; calving instruments)
biting insects? evidence inconclusive
proviral load is associated with transmission risk
BLV control
no vaccine available in US
test and slaughter, or test and segregate
PCR tests make it possible to identify and remove “super-shedders”
avoid procedures that can transmit infected lymphocytes (ex. do not reuse equipment; clean and disinfect between animals)
manage biting insects
FIV serotypes
at least 5 (A-E)
A & B predominate in US
animals can be superinfected with multiple subtypes
FIV transmission
primarily secreted in saliva
horizontal transmission → fighting and biting
prevalence higher among (intact) males than females
can also be transmitted in utero or through milk, particularly by acutely infected queens
(grooming, sexual contact NOT thought to be major modes of transmission)
FIV pathogenesis
replication kinetics similar to HIV/SIV → high acute viremia followed by long asymptomatic stage
decline in CD4+ T cells eventually leads to immunodeficiency
tempo & severity of disease may be dependent on age of animal and route of infection
FIV clinical signs
acute infection
nonspecific, “flu-like symptoms”: fever, malaise, lymphadenopathy
transient; not always apparent
may have long latent stage
chronic gingivitis, stomatitis
chronic upper respiratory disease, enteritis
opportunistic infections, including FeLV
FIV diagnosis
tumors are a common reason for FIV-infected cats to be brought in
presenting signs may also be similar to FeLV
IFA or ELISA → test for antibody against FIV
kittens may test false + for first ~12 wks due to maternal Ab
generally recommended to wait (or retest) at least 6 months after birth
FIV control
best control is prevention → avoid contact with free-roaming cats
vaccine no longer used in US
vaccinated animals seroconvert, confounding diagnostic tests
FIV strain variability is a major obstacle to effective vaccine development
equine infectious anemia virus (swamp fever) transmission
largely mechanical transmission by blood-sucking insects (tabanid & stable flies)
other modes, including iatrogenic possible
EIAV pathology
causes lifelong, persistent infection
acute infection may be clinically inapparent, though some animals have rapidly progressive disease
disease characterized by multiple interchanging syndromes; recurrent bouts of fever and illness
death occurs after many years of progressive, debilitating illness
EIAV clinical signs
7-21d incubation
acute onset of fever, mucosal hemorrhage, hemolytic anemia, fatigue, weakness, jaundice
high case fatality when acute signs apparent (up to 80%, particularly in foals?)
subacute: recurrent fever, anemia
recovered: no clinical signs, suitable for work
chronic: failure to thrive, fatigue, cachexia
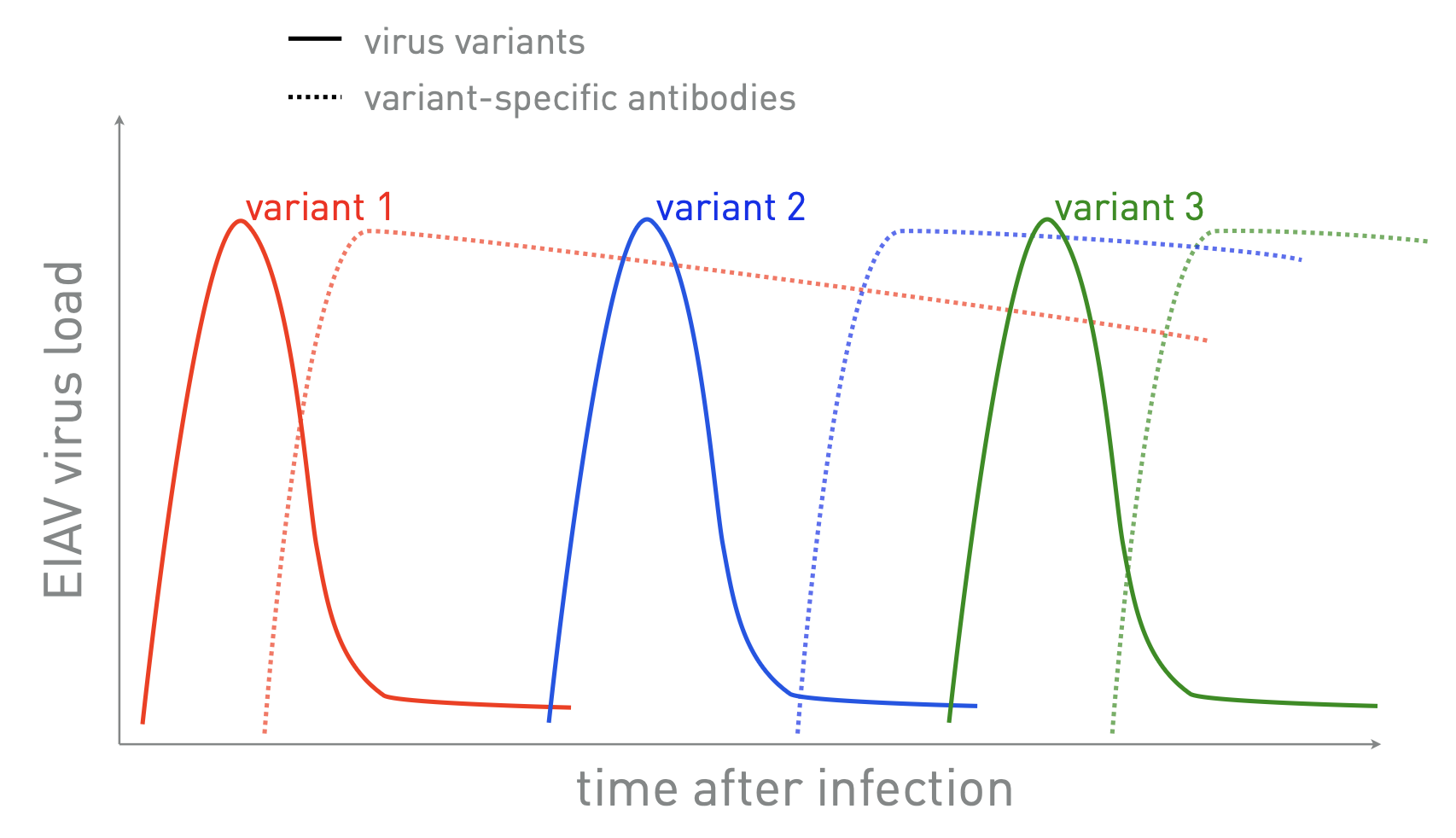
why does EIAV cause recurring bouts of illness?
within-host evolution → continually spawns novel antigenic variants that “escape” immune response
EIAV diagnosis
Coggins test: agar gel immunodiffusion (AGID) detects anti-EIAV antibody
being replaced with ELISA tests: faster, can perform in field
Western blot (confirmatory)
PCR
** negative Coggins or ELISA required to transport animals across state lines
in what region(s) of the US is EIAV prevalent?
southeastern US, texas, (and mississippi valley?)
EIAV control
spread controlled by euthanasia/isolation of infected animals & insect control
due to antigenic diversity, vaccines are not effective