(a) states of matter
1/16
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
solids in terms of the arrangement, movement and energy of the particles (1.1)
arrangement: particles are touching and packed close together in a regular pattern
movement: particles vibrate around a fixed point
energy: particles have less kinetic energy than liquids or gases

liquids in terms of the arrangement, movement and energy of the particles (1.1)
arrangement: particles are touching but irregularly
movement: particles are free to move i.e. to fit the shape of their container
energy: particles have less kinetic energy than gases but more than solids do
gases in terms of the arrangement, movement and energy of the particles (1.1)
arrangement: particles are far apart and there are no force between them
movement: particles are free to move i.e. to fit the shape of their container
energy: particles have more kinetic energy than liquids and solids
melting (1.2)
particles gain kinetic energy and vibrate faster, allowing particles to overcome the forces of attraction that hold them together in the solid. the regular pattern of the solid is broken down and particles can now slide past one another and change neighbours.
freezing (1.2)
cool the liquid until it freezes. particles lose kinetic energy and allows the forces of attraction between the particles to hold them together. particles arrange themselves into a regular pattern and are no longer able to slide past one another.
evaporation (1.2)
heat the liquid until it boils. particles gain kinetic energy and move further apart causing the forces of attraction between the particles to be completely broken and they are able to escape from the liquid.
condensation (1.2)
cool the gas until it condenses. particles lose kinetic energy and vibrates faster, allowing the forces of attraction between the particles closer together. the particles eventually clump together to form a liquid.
sublimation (1.2)
heat the solid until it sublimes. particles gain kinetic energy and vibrates faster. this causes the forces of attraction between the particles to be completely broken and are able to escape from the solid.
dilution of coloured solutions (1.3)
dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
the random motion of particles cause the purple colour to eventually be evenly spread out throughout the water.
adding more water to the solution causes the potassium manganate(VII) particles to spread out further apart therefore the solutions becomes less purple. this is called dilution.
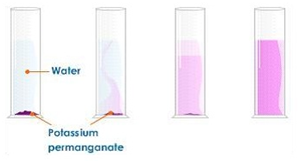
diffusion experiments (1.3)
when ammonia gas and hydrogen chloride gas mix, they react together to form a white solid called ammonium chloride.
ammonia + hydrogen chloride –> ammonium chloride
NH3(g) + HCl(g) –> NH4Cl(s)
a cotton wool pad was soaked in ammonia solution and another was soaked in hydrogen chloride solution. the two pads were then put into opposite ends of a dry glass tube at the same time.
the white ring of ammonium chloride forms closer to the hydrochloric acid end because ammonia particles are lighter than hydrogen chloride particles and therefore travel faster.
even though these particles travel at several hundred metres per second, it takes about 5 min for the ring to form. this is because the particles move in random directions and will collide with air particles in the tube.
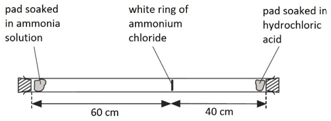
solvent (1.4)
when a solid dissolves in a liquid, the liquid is called the solvent
solute (1.4)
when a solid dissolves in a liquid, the solid is called the solute
solution (1.4)
when a solid dissolves in a liquid, the liquid formed is called the solution
saturated solution (1.4)
a saturated solution is a solution into which no more solute can be dissolved
solubility (1.5C)
the measurement of how much of a substance will dissolved in a given amount of liquid. it can be expressed in g per 100g of solvent
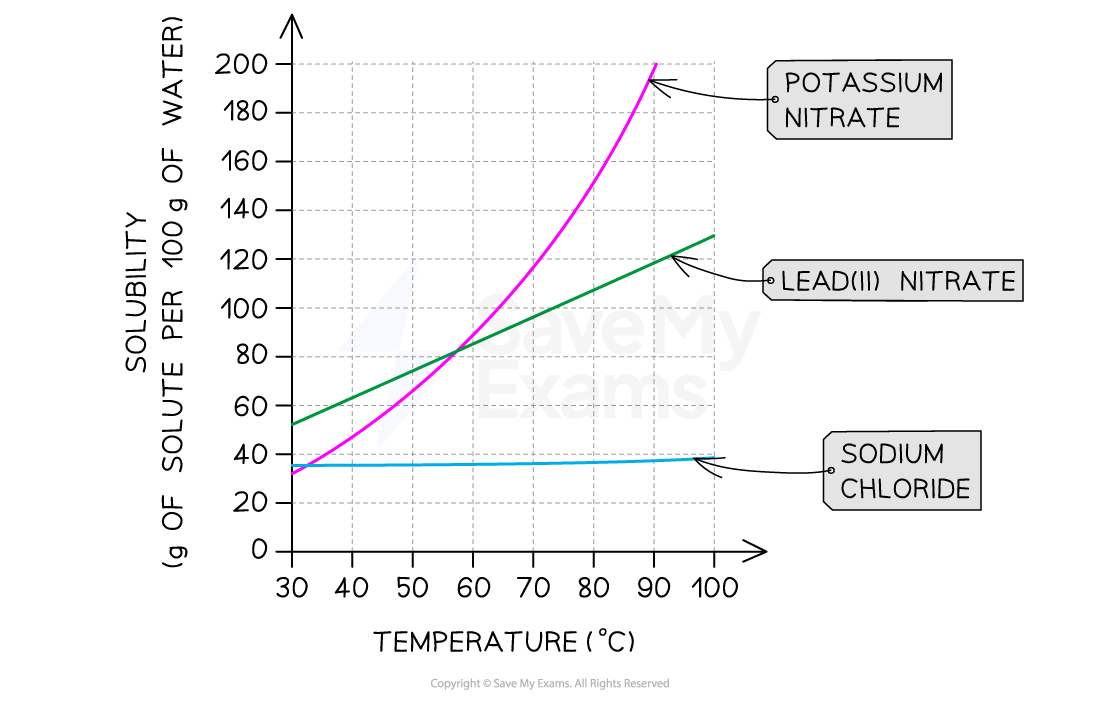
solubility curves (1.6C)
solubility graphs or curves represent solubility in g per 100 g of water plotted against temperature
to plot a solubility curve, the maximum mass of solute that can be dissolved in 100 g of water before a saturated solution is formed, is determined at a series of different temperatures
investigate the solubility of a solid in water at a specific temperature (1.7C)
at a chosen temperature (e.g. 40⁰C) a saturated solution is created of potassium nitrate (KNO₃) for example.
some of this solution (not any residual solid) is poured off and weighed. the water is then evaporated from this solution to leave a residue of potassium nitrate which is then weighed.
the difference between the two measured masses is the mass of evaporated water.
the solubility, in grams per 100g of water, is equal to 100 times the mass of potassium nitrate residue divided by the mass of evaporated water.
solubility (g/100g) = mass of solute / mass of solvent * 100