Science mass, gravity, volume, metric conversions test
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Metric conversion
Express certain measurements in easier terms putting one measurement in terms that you are familiar with.
Meters
Distance
Liters
Volume
Grams
Mass
1 step=
1 decimal place movement
Example: 500 KG =___MG
500,000,000 MG
Example: 4.5Mg=____Ng
4,500,000
The mass of an astronaut is 110kg on Earth, what happened to his mass when he landed on the moon Explain:
Nothing happened to the astronauts mass, mass stays the same no matter where you are in the universe, what did change was his weight.
What is volume
The amount of liquid or space an object takes up( or is )
Volume is not
3D while area= 2D
There are 3 ways to measure volume
Irregular shaped solids-water displacement
Regular(geometric) LxWxH
Liquids graduated cylinder
1ml=_____
1cm3 (cubed)
What is mass?
The amount of matter in an object
Mass is measured using a :
Triple beam balance (or just balance)
Mass is measured in
Grams or Kilograms
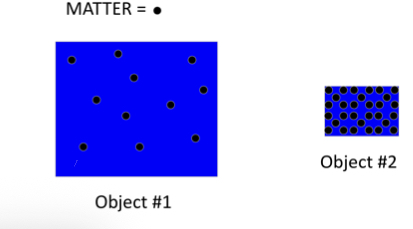
Which object is more massive? Which object has more mass?
Having more mass and being massive is the same thing, in this case object #2 is more massive and has more mass.
What is gravity?
Gravity is the force of attraction between two objects with mass
The strength of Gravity depends on:
Distance and mass. Distance is more important than mass when talking about gravity.

What is weight?
Weight is the gravitational pull on an objects mass.
Mass Vrs weight

Density
The compactness of matter in a given space
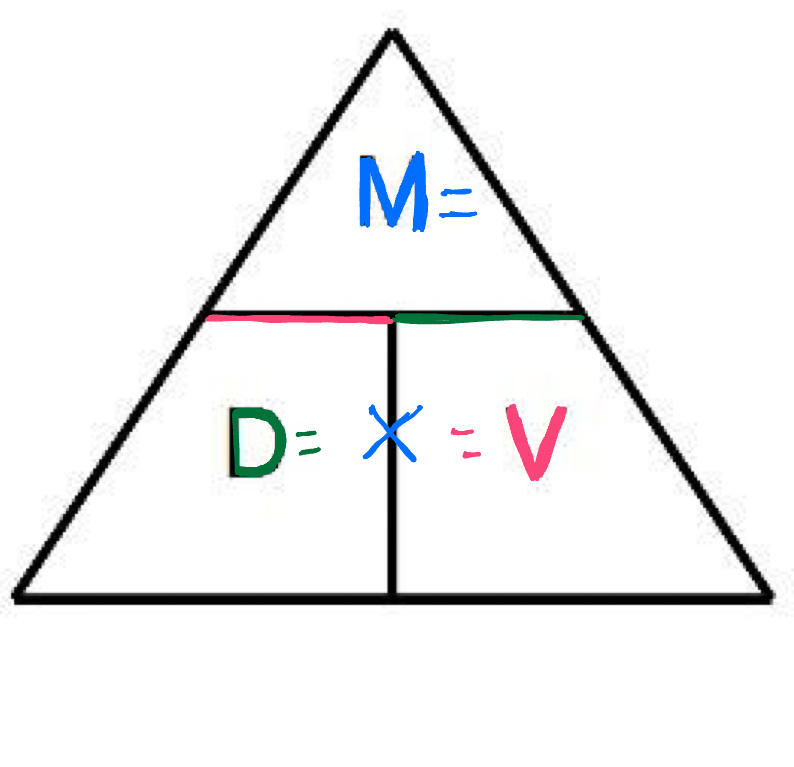
Density formula:
D=M(mass)/V(volume)
Practice Density formula questions:
D= 0.6 g/cm3 M= 54g——- V= 90cm3
D=1.0 g/cm3 V= 4.0 cm3—— M= 60g
Separation
Different materials separate/organize each other by density
The density of a pure substance
Always stays the same
Ex. Gold 19.3 g/cm3– always stays the same not matter the amount of cold
Density of water
Maximum- 1.0 g/ml @ 4 degrees Celsius
Density of water is odd because
Most substances are most dense when they are a solid
Three states of matter
Solid, liquid, and gas
Maximum and minimum densities
Maximum density usually occurs in the solid state
Minimum density usually occurs in the gas state
****exception water is most dense at 4 degrees Celsius, which is the liquid state.
We can determine the density of an object by placing it in water: steps
1.) place object in water
2.) estimate the percentage of the object that is submerged in the water
3.) multiply percentage by the density of water
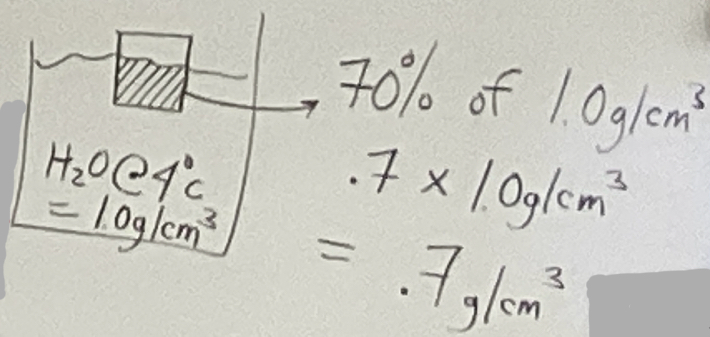
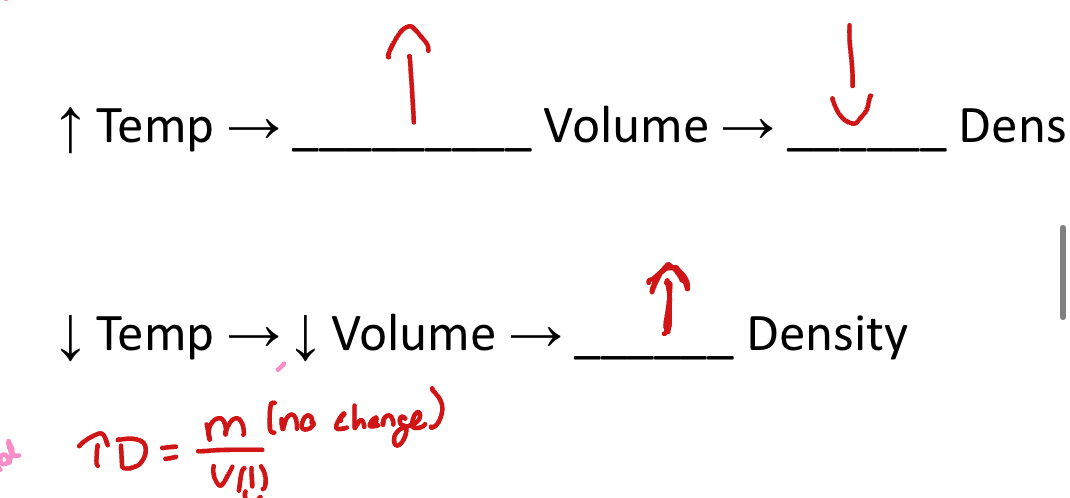
Temperature
A change in temperature can change density( heat makes objects expand)
Temp goes up, volume goes up, density ones down
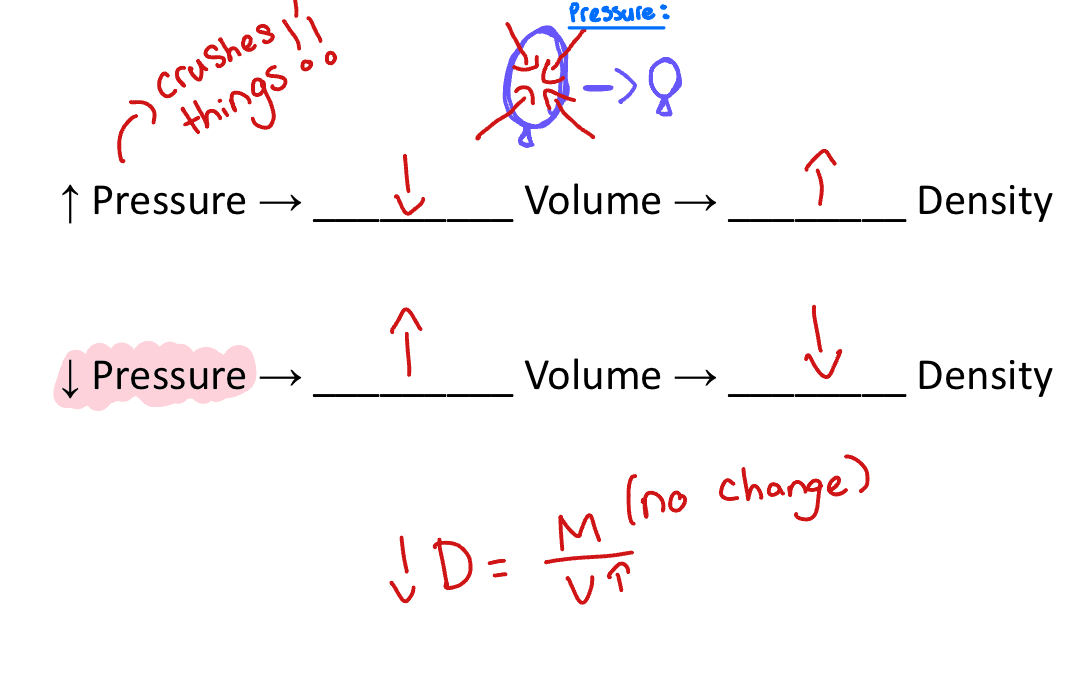
Air pressure
High air pressure= lower volume= higher density
Low air pressure= higher volume= lower density
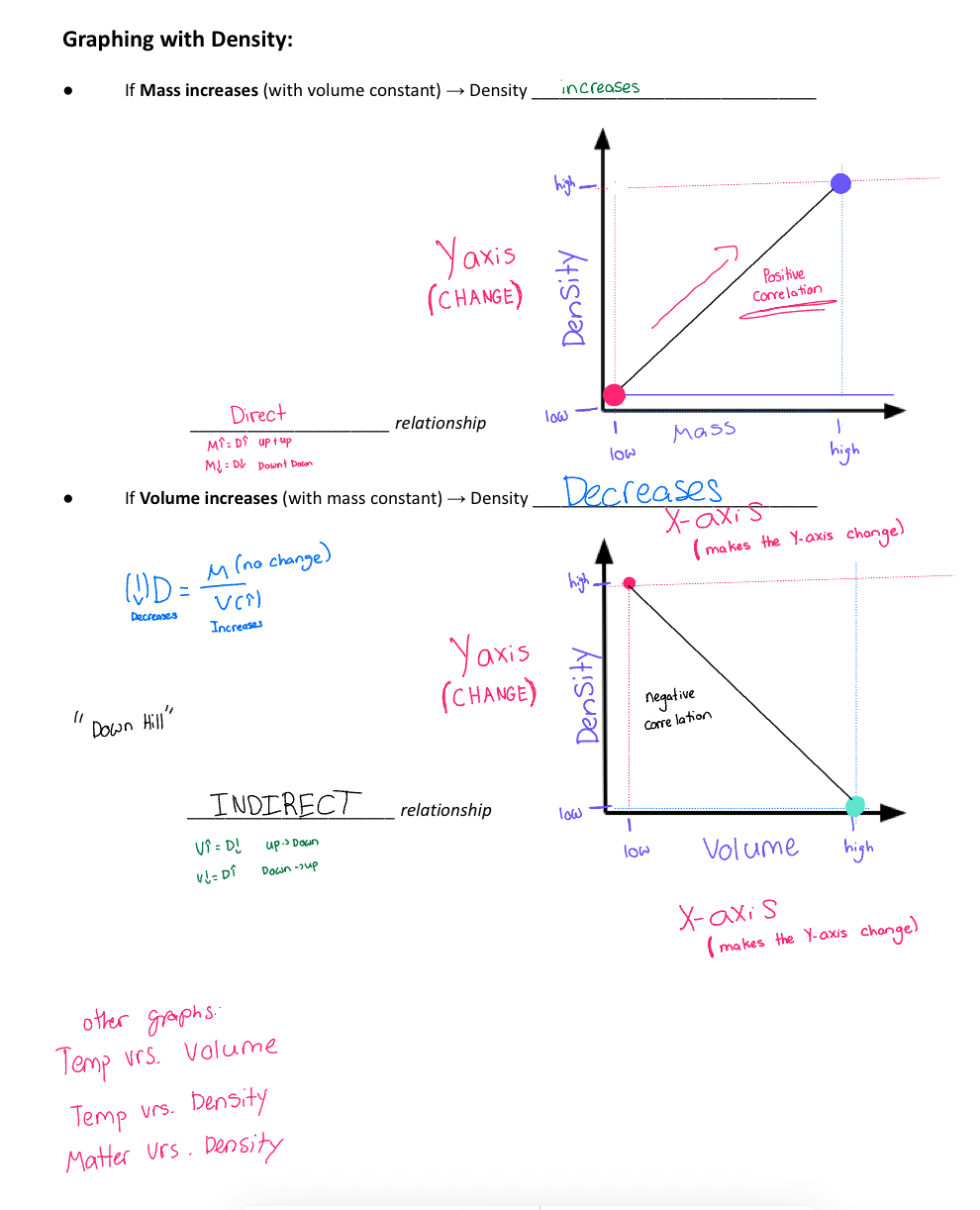
Graphing with density: