Immunology Diagnostics AHS
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
MHC properties (3)
Bind a variety of antigens but not all, combinations of gene products
Multiple loci
High polymorphism
Granulocytes:
Neutrophil
Eosinophil
Basophil
Inflammation, segmented nuclei, forms pus when dies, roles (degranulation, phagocytosis, neutrophil extracellular traps)
Parasitic infection and allergic reaction, degranulation for larger targets
Allergic reaction, activated by IgE cross-linkage, release histamine (increased vascular permeability, smooth muscle contraction)
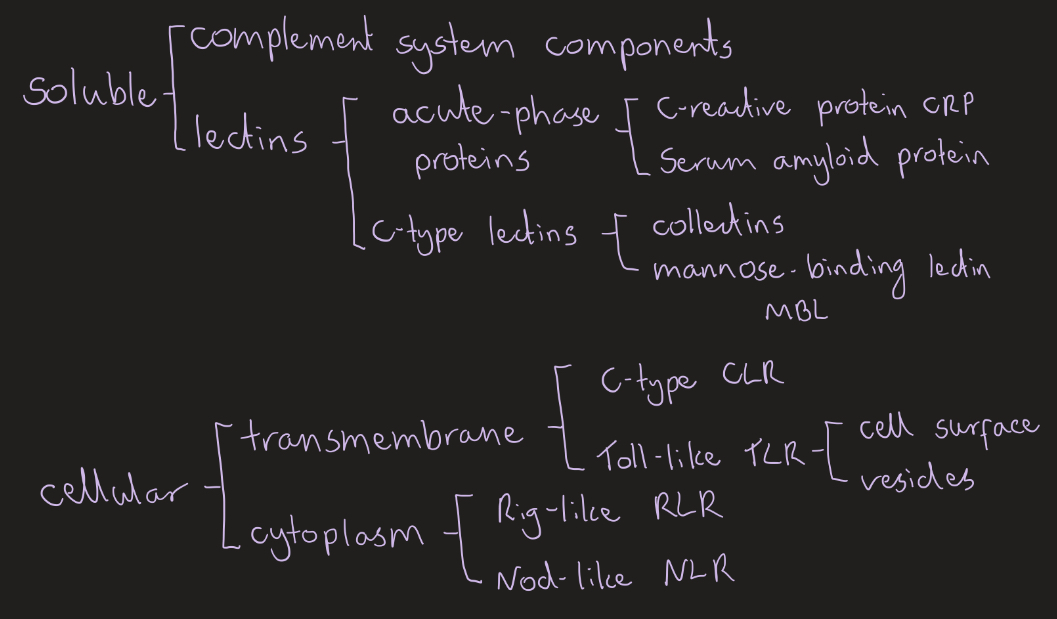
Pattern recognition receptors:
Soluble PRRs
Cellular PRRs
Soluble- extracellular, bind and tag
Cellular- intracellular, trigger immune response
Complement system:
Features (4)
Outcomes (4)
Regulation (3)
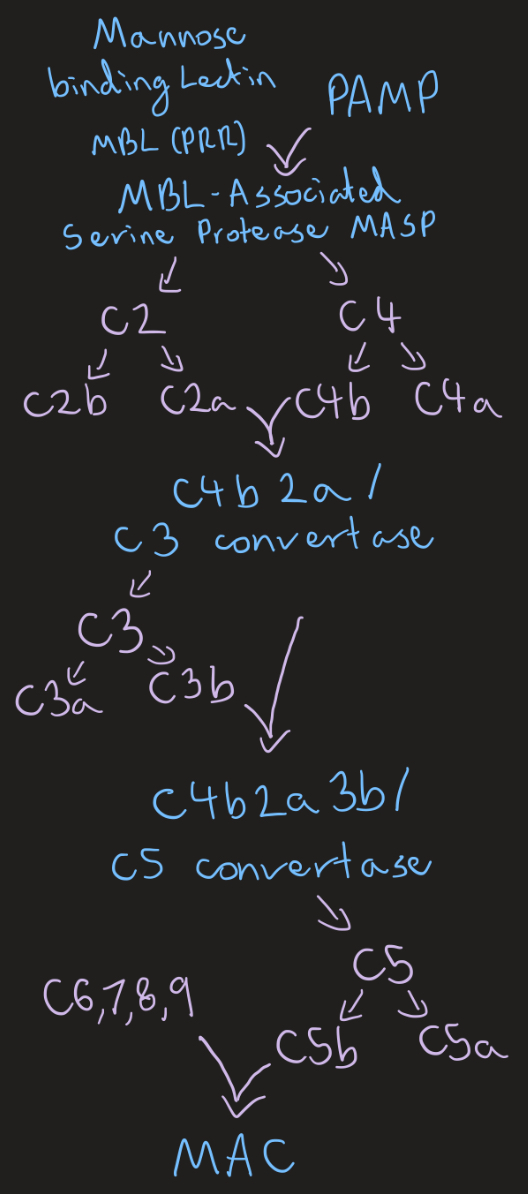
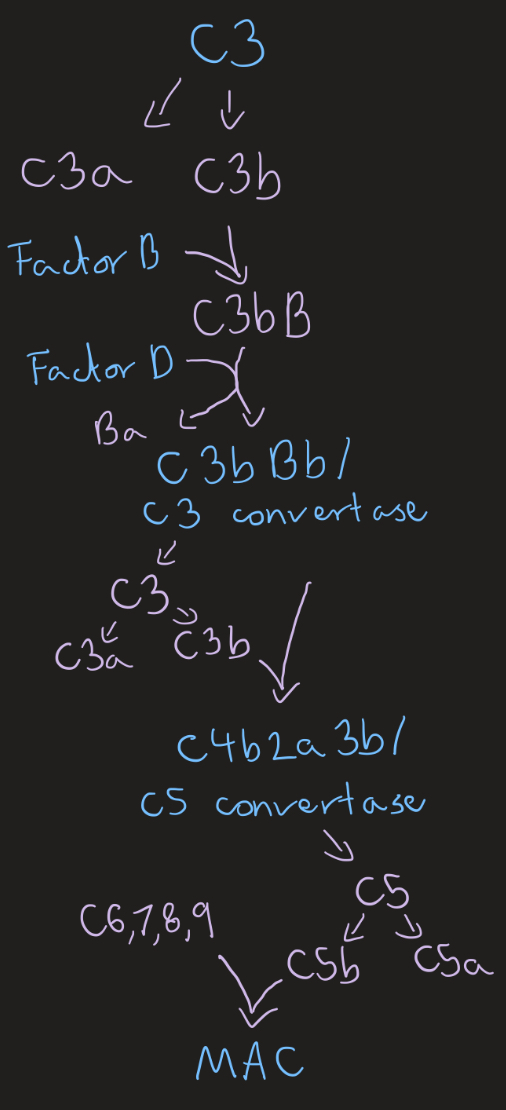
Liver synthesis, single amplification, C3 and C5 convert are creation, common terminal pathway MAC
Opsonisation (phagocytes recognise C3b), inflammation induction (C3a and C5a attract immune cells, mast cell degranulation), clearance of immune complexes/ antigen-antibody complexes, MAC killing
Proteins increase selectivity and limit cascade continuing (by inhibiting enzyme activity or promoting degeneration), factor H and I bind C3b on host cells and inhibit, C1 inhibitor stops unwanted classical pathway activation
Extravasation/ Diapedesis of neutrophils (3)
Rolling- selectin mediated, along endothelial cell surface
Firm adhesion and crawling- integrin mediated, to endothelial surface
Emigration and chemotaxis- swarm into tissue towards chemoattractants
Antigen properties to stimulate immune response (5)
Types of antigens (2)
Complex
Organic
Degradable (protein)
Large
Recognisably foreign
Exogenous (viruses, allergens, micro-organisms)
Endogenous (viral infection consequences, cancer cells, autoantigens/self-antigens )
Classic complement pathway
Triggered by antibody/ antigen complexes, C3b opsonisation, C3a and C5a are pro-inflammatory mediators

Lectin complement pathway
Triggered by lectins
Pathogen associated molecular patterns PAMPs (5)
Damage associated molecular patterns DAMPs (3)
Abundant, essential molecules, structurally conserved, shared by entire pathogen class, detected by PRRs
Molecules escaping dead, dying, or damaged cells, intracellular (cellular components), extracellular (released or modified)
Aspects:
Barriers (2) (4)
Innate immunity (2)
Adaptive immunity (2)
Epithelial barriers (skin, mucosal), humoral innate immunity (enzymes, iron chelators, antimicrobial peptides, complement proteins)
Cellular innate immunity, inflammation/ mediators
Cellular components (B/T lymphocytes), humoral components (IgG, IgM, IgA, IgE)
Membrane attack complex MAC (3)
Complement system end product
C5b, C6, C7, C8, C9 component complex
Forms protein complex, hole in membrane, death by osmotic lysis
Sentinel cells:
Mast cells
(Monocytes)
Macrophages
Dendritic cells
Long-lived, tissue-resident, parasitic infection and allergic reaction, releases histamine
(Short-lived, blood, single kidney nucleus, differentiates to macrophages and dendritic cells)
Repeat phagocytosis (activity increased by interferon gamma IFN-y from T and NK cells), produce IL-1 IL-6 TNF-a, APC initiating adaptive immunity
Immature (tissue, antigen uptake), mature (lymph node, APC initiating adaptive immunity)
Natural killer cells (NK cells) (4)
Blood and lymphoid tissue
Cytotoxic granules
Release IFN-y to activate macrophages
Activated when MHC 1 not present
Alternative complement pathway
Spontaneous C3 hydrolysis, Factors H and I inactivate and degrade C3b when bound to host cells due to sialic acid present
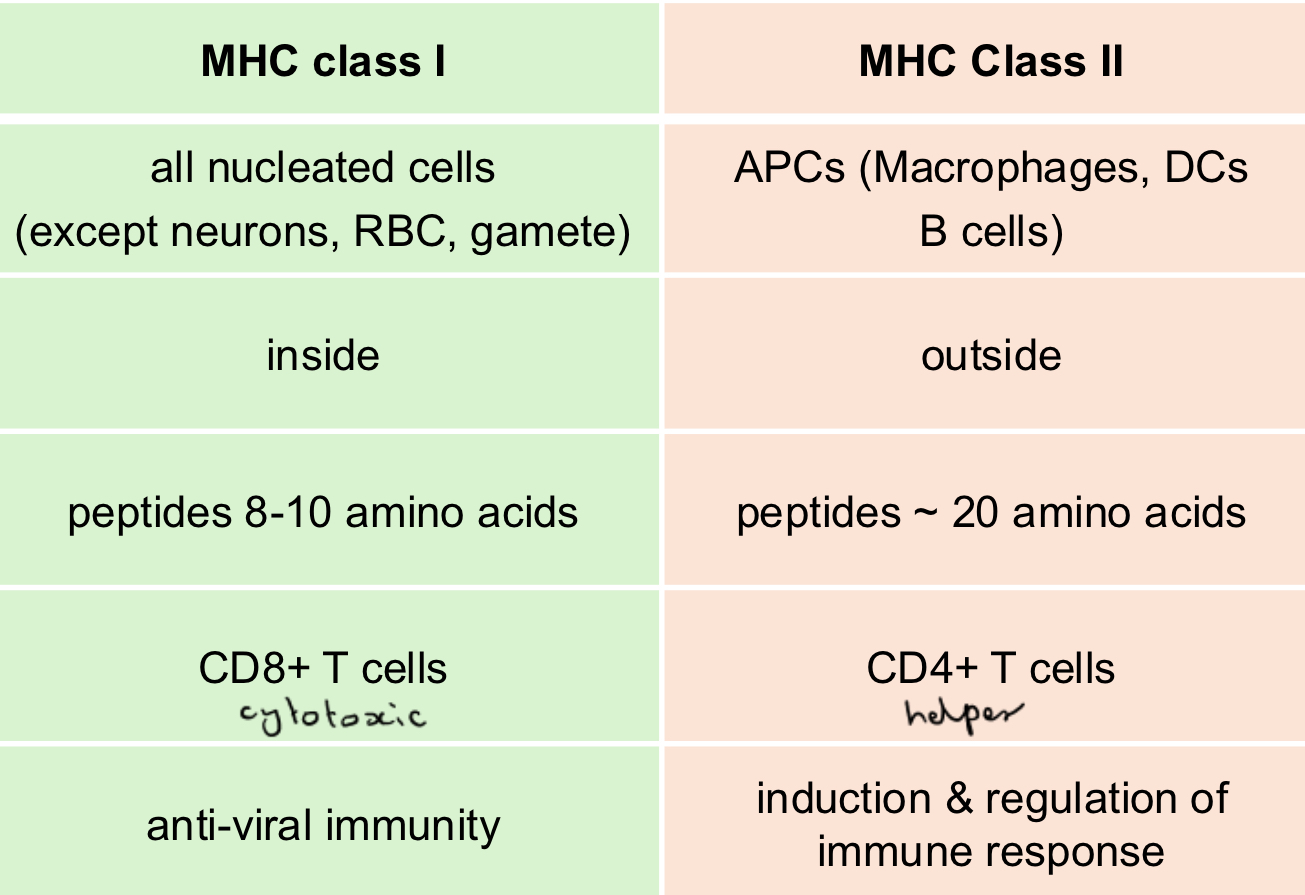
MHC both
MHC class 1
MHC class 2
(Expressed by, antigen from, bind, recognised by, main function)
Two chain molecule, transmembrane glycoprotein, bind multiple antigenic peptides
Phagocytosis (4)
Recognition and attachment
Engulfment forms phagosome
Killing and degradation of phagolysosome
Reactive oxygen species produced more in activated macrophages
Inflammation:
Acute stages (3)
Mediators (3)
Vascular/fluid phase (vasodilation, increased permeability)
Cellular phase (leukocyte recruitment)
Resolution phase (cells removed, tissue repair, or chronic)
IL-1, IL-6, TNF-a (initiate adaptive)
Histamine, eicosanoids/ lipid mediators (permeability)
C3a and C5a/ anaphylatoxins (mast cells degranulation, smooth muscle contraction)
Features of adaptive immunity (4)
Specific (receptor, antigen, cell signalling, selective activation, clonal expansion)
Adaptation (quality and quantity, modulate receptor specificity)
Memory (shorter lag time, improved specificity, basis of vac)
Discrimination (self vs non-self, central- primary lymphoid organs in immature lymphocytes and peripheral tolerance- secondary lymphoid organs in mature lymphocytes )
B cells
Produce and mature in bone marrow
Recognise linear and 3D antigens in secondary lymphoid tissues
Recognition, present to T helper, clonal expansion, differentiation, plasma cells release antibodies, class switch regulated by T helper cytokines, affinity maturation for memory cells (germinal centres)
Antibody function (6)
Blockade by IgM and IgG- bind to bacteria adhesion, doesn’t bind to cell receptor, neutralisation
Agglutination by IgA- prevent attachment, neutralisation
Opsonisation by IgG- tag for phagocytosis via FcR
IgE mediated mast cell degranulation- degranulation after antigen contact via antibody
Worm expulsion by eosinophils and IgG- antibody binds to parasite, eosinophils bind to antibody, antibody-dependent cytotoxicity ADCC
Complement activation- cell lysis, enhanced phagocytosis, inflammation
T cells
Produced in bone marrow (immature)
Mature but naive in thymus (test receptor, reactivity to self, expression of CD4 or CD8)
Activation in secondary lymphoid tissue (antigen recognition, co-stimulation, cytokines from APC)
Regulation of adaptive immunity (CD4+, to Th1 or Th2, MHC 2)
Cytotoxic cell killing (CD8+, MHC 1)
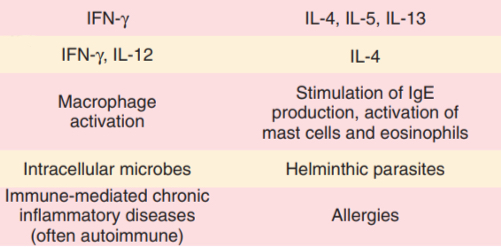
Th1 and Th2 (CD4+)
(Cytokines produced, cytokines that induce subset, immunological reaction triggers, host defence against, role in disease)
Cytotoxic T cells (CD8+) function (4)
Interact with antigen MHC 1 via TCR
Binding triggers degranulation
Perforin forms pores in target cell
Granzymes insert via pore and induces apoptosis
Structure:
Both
BCR (somatic recombination)
TCR (rearrangement)
Constant region (function), variable region (antigen binding site), transmembrane glycoprotein
Whole molecule binding, one Fc domain, two binding sites, specificity via variable domain in bone marrow, D joins J, DJ joins V, VDJ expressed with constant region m to give antibody IgM, class switching to IgG IgA IgE (T cell help) in secondary lymphoid organs
MHC presented antigen binding only, alpha and beta or gamma and delta chains, rearrangement in thymus
Antigens: type, function
Tolerogenic
Immunogenic
Factors affecting (4)
Self antigens, may induce anergy and apoptosis
Foreign microbial antigens, induce immune response which eliminates
Nature, dose, administration route, co-stimulators signal presence
Autoimmune diseases:
Disorder of, activation of, response to
Mechanisms (3)
Classification (2)
Factors effecting (3)
Adaptive response, autoreactive lymphocytes, self proteins/ autoantigens
Immune complexes (hypersensitivity type 1, systemic), circulating antibodies (hypersensitivity type 2 and 3, organ specific), autoreactive T lymphocytes (hypersensitivity type 4, organ specific)
Organ-specific autoimmunity (restricted antigen in tissue/cell, Immune-Mediated Hemolytic Anaemia against RBC surface, Myasthenia against ACh receptor), Systemic autoimmunity (not restricted to organ/tissue, Systemic Lupus erythematousus, deposition of autoantibody-autoantigen complexes in tissues)
External triggers (chemicals, infections), hormonal influences, genetic predisposition
Autoimmune disease development (4)
Mechanisms of activation of autoreactive lymphocytes
Autoreactive lymphocytes escapes elimination and moves to periphery, encounters specific self antigen, peripheral tolerance responses fail, response results in clinical damage
Inflammation, molecular mimicry (pathigen resembles self antigens), defects in immune system components, epitope spreading (previously hidden antigen)
Immunodeficiency diseases:
What
Congenital/ primary (innate 3, adaptive 3)
Acquired/ secondary AIDs (infectious-induced 2, non-infectious-induced 4)
Increases susceptibility to infections
Genetic mutation or mothers exposure, at birth
-phagocytosis defects, complement deficiencies (C3), leukocyte adhesion deficiency LAD
-humoral immunoglobulin deficiencies (IgA), cellular cell-mediated immunity deficiencies, sever combined immunodeficiency diseases SCIDs
Extrinsic causes, later in life
-secondary phagocytosis deficiencies, viral agents (FIV)
-latrogenic (another disease or treatment complication, drug-induced granulocytopenia), idiopathic granulocutopenia (low granulocytes without known cause), malnutrition, splenectomy
Hypersensitivities:
What
Stages (2)
Causes (3)
Secondary responses that cause harm, excessive or abnormal, antigen specific
-Sensitisation stage (primary immune response to antigen)
-Effector stage (harmful secondary response upon re-exposure)
Reactions against nonmicrobial environmental antigens, microbes, self-antigens (autoimmunity)
Type 1 hypersensitivity/ immediate (allergies):
What, time, type (2, examples)
Mechanism (4, 3)
Mediators (5)
Antibody-mediated (IgE agains certain environmental allergens), quick (1-30min), allergy (allergen), atopy, localised responses (anatomical location, based on entry route- hay fever, atopic dermatitis/eczema, food allergy), systemic response (anaphylaxis, large quantity of inflammatory mediators and vasodilators released)
-Sensitisation: APCs, Th2 cells activated, release cytokines attract WBCs, plasma cells class switch to IgE, bind to basophil/ mast cells
-Activation/effector: allergen binds to IgE, early phase reaction (re-exposure, binds to IgE on mast cell, degranulation triggered, mediators/ histamine released tissue-specific symptoms), late phase reaction (cytokines attract and activate eosinophils, release substance causing tissue damage, macrophages and neutrophils arrive release inflammatory mediators, ongoing inflammation and tissue injury)
Mast cell, histamine, platelet-activating factor, leukotrienes, prostaglandins (increased vascular permeability, mucus secretions, fluid secretion and peristalsis)
Type 2 hypersensitivity/ cytotoxic (autoimmune diseases):
What, time, target
Mechanism (2)
Haemolytic anaemias (2)
Antibody-mediated (IgM or IgG, bind directly to antigen on cell surface, cell lysis), quick (5-6hrs), target mobile (in bv) or fixed cells (in tissue), foreign or self antigen
-Sensitisation: antigen exposure, APC, IgM and IgG production from plasma cell
-Activation/effector: IgM or IgG bind antigen on cell surface, activate classical complete pathway (MAC and cell lysis), Antibody-dependent cellular cytotoxicity/ ADCC (antibodies coat and tag cell, Fc regions bind, degranulation of cytotoxic granules from immune cells), phagocytosis (macrophages, neutrophils, NK cells)
Lytic destruction on RBCs
- Alloimmune (antibodies against foreign RBC interact with allogeneic RBC, blood transfusions, haemolytic disease of newborn via colostrum)
-Autoimmune (antibodies against own RBCs, Immune-Mediated haemolytic anaemia, intravascular/ complement-mediated, extravascular/phagocytosis in liver and spleen, anaemia/jaundice/splenomegaly, saline agglutination test, Coombs test, spherocytosis)
Type 3 hypersensitivity/ soluble immune-complex:
What, time
Mechanism (6)
Types (2)
Antibody-mediated (classical complement system fails to phagocytose and clear, C3b deposition fails to regulate size), quick (4-6hrs)
IgG or IgM bind to soluble antigens, form insoluble immune complexes ICs, ICs not phagocyted get stuck in bv and tissues and activate complement, anaphylatoxins attract and activate mast cells which mediators secreted, increased vascular permeability, tissue cells with C3b destroyed by neutrophils/macrophages
-Localised: Arthus reaction (immune complexes deposited within localised sites and tissue), Hypersensitivity pneumonitis (alveoli), blue eye/anterior uveitis (cornea)
-Generalised/systemic: serum sickness and systemic lupus erythematosus (ICs deposited in bv in joints, capillaries and renal glomeruli) sites far away from original, multiple sites affected
Type 4 hypersensitivity/ delayed DTH (T cell-mediated):
What, time
Types (2, mediated by)
Results from tissue-damaging actions of effector Th cells, CTLs, macrophages, slower (24-72hrs)
-Chronic delayed-type hypersensitivity (antigen derived from agents unusually resistant to elimination, tuberculosis), CD4 Th1 (IFn-y release, macrophage activation for phagocytosis or granuloma formation)
-Contact hypersensitivity/ dermatitis (response to molecule bound to self protein in uppermost skin layer, poison ivy rash), CD8+ CTL (directly attack cell)
In house diagnostics eg
Diagnostic lab eg (why)
Complete blood count, blood tests, stool sample, rinse sample, smears, basic microscopy
All in-house, additional biochemistry, advanced microscopy, antibiotic sensitivity, microbiology (Equipment, protocols, personnel, expertise)
Microbial diagnostic:
Specimen collection (4)
Indirect method (1)
Direct methods-
Microscopy (2) :Staining (3)
Non-culture technique (2)
Culture technique (5)-
Isolation on culture media/ Identification (4, 2-4)
Collected before antibiotics, representative of infection site, sterile manner, transported properly and quickly
Antibody detection
Stained/morphology: size shape arrangement (cocci, bacilli, other, appendaged), staining, spores (shape, position, bulging), capsule; Unstained/motility
-Simple: indentify more clearly,methylene blue
-Differential: distinguish differences in mixed culture, gram stain, +purple -pink, Ziehl-Neelsen/ acid fast
-Special: stain specific features, flagellar stain
Genetic analysis, antigen detection
Culture media (solid agar, liquid broth), colony culture (form, elevation, margin), differential culture media (exploits different metabolic capabilities), quantitation (diluting for less colony forming units), culture conditions
- Microscopy
-Biochemical tests: oxidative-fermentative test (anaerobic fermentation/ yellow or aerobic oxidation/ green), catalase test (decomposition of hydrogen peroxide to oxygen/bubbles and water), oxidase test (catalyse electron transfer colourless to purple), API strips (miniature tests on one analytical profile index strip, profile based on bacteria)
-DNA probe
-Serology
Antimicrobial susceptibility testing:
Should be (3)
Methods of sourcing material (2)
Methods of AST (3)
Standardised, include the most appropriate antimicrobials (antibiotics, antivirals, antifungals, antiparasitics), use the most appropriate methodology
-Direct method: pathological specimen, used to inoculate a culture plate, single colonies selected to inoculate liquid culture
-Indirect method: pure culture already isolated, sensitivity plate created directly
Disk diffusion/ Kirby-Bauer method: solid agar plate, discs with known conc antimicrobial agent, pure culture to be tested, anything sensitive doesn’t grow/ zone of inhibition/ minimum inhibitory concentration MIC (lowest conc, inhibit visible growth)
Dilution: broth or agar, series of dilutions with range of strengths of antimicrobials, small amount on microbe added and incubated, lowest conc with no visible growth identifies MIC,
(Both: Etest, strip with dif concs of antimicrobial down; Molecular: allows identification of pathogen possesses antimicrobial resistance gene)
AST and Antimicrobial Stewardship AMS:
What (4)
Role (4)
Challenges (4)
Coordinated interventions to improve and measure appropriate use of antimicrobials, minimise resistance, optimise clinical outcomes, reduce adverse effects and costs
Use AST to chose targeted antibiotics, preserve antimicrobial efficacy, improve treatment and patient outcomes, contribute to surveillance and monitoring
Time delay, application of clinical breakpoint, some pathogens can’t be cultured, client expectation
Types of microscopes (3)
Compound light microscope: two lenses (ocular in eyepiece, objective), beam of light to view specimen, Kohler illumination/ optimal set up (iris, diaphragm of lamp, specimen and primary image are simultaneously in focus), used in bacterial smears blood smear histology slides swabs fine needle aspirates
Dark-field microscope: scatters light causing it to reflect off specimens at angle, bright specimen on dark background, live spirochetes
Phase-contrast microscope: light diffracted and shifted in phase by specimen (phase objective), transformed by phase contract into amplitude differences, live organisms, visualisation of transparent cells and structures without stains
Saline/Slide Agglutination Test SAT (purpose, procedure, interpretation)
Direct Agglutination Test DAT/ Coombs test (purpose, procedure, interpretation)
Initial detection of autoagglutination of RBC in dogs with IMHA due to autoantibodies binding RBC; drop of blood on slide, drop of saline, gently rock, observe for clumping (macro/micro); visible clumping supports IMHA diagnosis, false negatives if low antibody levels for visible agglutination, false positive if rouleaux detected (add saline should separate)
Detect presence of antibodies or C3 on RBC and support IMHA diagnosis; centrifuge blood tube, wash and centrifuge 3 times, add top buffer to blood, insert DAT membrane; positive or negative on test strip to support, false negative if not enough autoantibodies or weak affinity
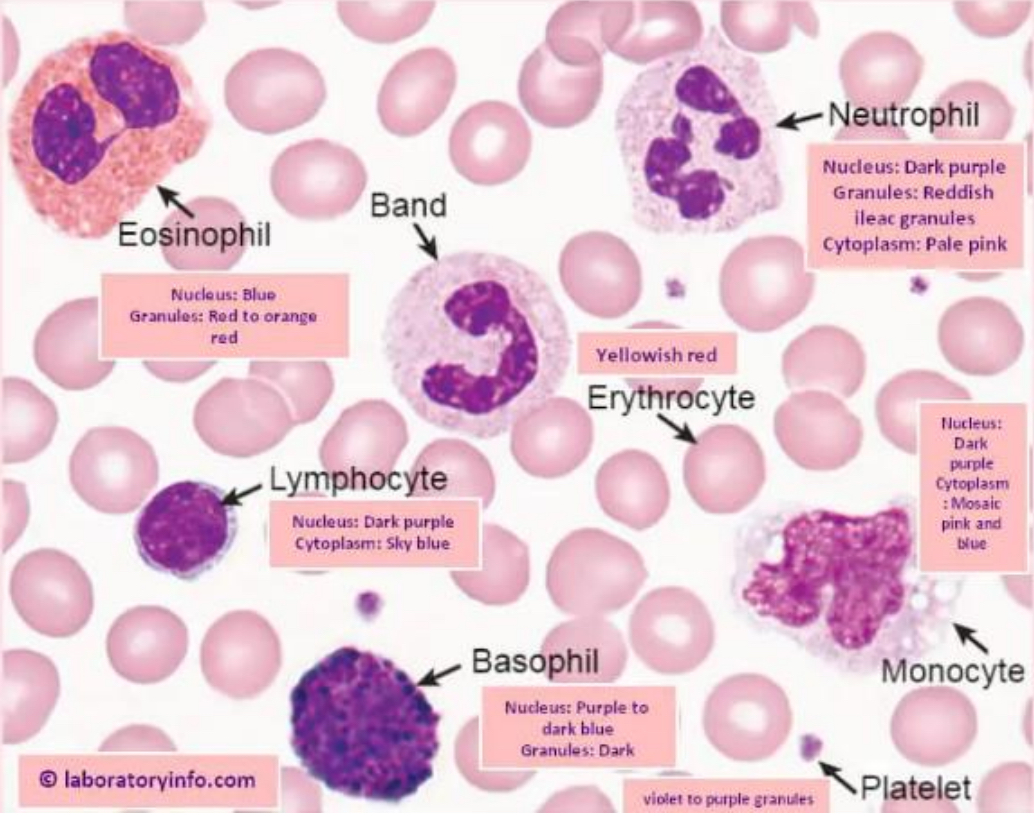
WBC types:
Granulocytes (neutrophil, eosinophils, basophils)
Agranulocytes (lymphocytes, monocytes)
Platelets
Leukogram
Arneth index
Common, granules, pale cytoplasm, 3-5 lobe segmented nucleus, 2-3 times RBC size
Increase in parasitic infections, 2 equally-segmented nucleus, orange/red granules
Rarely seen, blue/purple granules, 2-3 times RBC size, hard to see nucleus
No granules, no nucleus segmentation, dense nucleus, less cytoplasm than monocytes, almost same size RBC
Blue/grey cytoplasm with no granules, round kidney shaped nucleus no segmentation, 4-5 times RBC size, white cytoplasmic vacuoles
No nucleus, small and clustered, blue/purple cytoplasm
Total and differential leukocyte count (stress, physiological, inflammatory, leukemia), from monolayer
Classification of neutrophils based on nuclear lobe nr, assess neutrophil maturity, left shift due to inflammation shows immature neutrophils in blood
Immunodiagnostics tests: How
Immunoassays: How
Use an antigen-antibody reactions to detect and identify a specific antigen or antibody associated with a disease-causing organism (detects antibodies/exposure or antigens/infection)
Detect and measure specific immune responses to substances, test for antigen antibodies/levels of antibodies for stage of disease or protection level
Lateral flow immunoassay (LFD):
Types
How it works
Example
Advantage
Disadvantage
Sandwich or competitive assay
Sample onto sample pad, travels laterally through conjugate pad (labelled reagent and antibodies which release into the sample) onto test membrane/zone of detection captured by immobilised antibodies
Canine heartworm antigen rapid test (sandwich) in blood
Rapid, portable, cheap, accessible
Reduced sensitivity, qualitative, false positives cross reactivity of similar antigens
Emzyme-linked absorbent immunoassay (ELIZA):
Types
Example
Advantage
Disadvantage
Antigen detection (virus, bacterium, protein, hormone) as sample (plasma, blood, saliva, urine, CSF), add specific primary antibodies, add enzyme-linked secondary antibodies, add substrate, look for colour change
Antibody detection, add purified antigen, add sample (blood, plasma), add enzyme-linked secondary antibodies, add substrate, look for colour change
Serial binding on a multi-well plate, SNAP test, qualitative (presence) or quantitative (stage of disease) ELIZA
High sensitivity, high specificity, qualitative and quantitative, scalable, adaptable, safe
Time, skilled expertise, reagent storage, costly, sample quality, human error
Agglutination immunodiagnostic:
What
Example
Advantage
Disadvantage
Antibodies binding to antigens and clumping, micro/macroscopical viewing, screening as qualitative
Blood typing, immuno-mediated disease, bacterial identification
Rapid, field use, cheap, accessible, screening option
Reduced sensitivity and specificity, false positives, limited quantitation
Precipitation immunodiagnostic
Agar gel immunodiffusion (AGID)
(What, advantage, disadvantage)
Based on antibody antigen interactions, two soluble reactants in optimal portions, make one visible insoluble product
-Rapid, high specificity, cheap, screening, confirmatory in combination
-Low sensitivity, lab setup, labour intensive, interference (improper pH, temp)
Based on antigen antibody migration towards each other through specific medium, positive shows opaque precipitant line between antiserum and antigen wells, detect antibodies to fungal pathogens and Brucella canis
Complement fixation: How
Immunofluorescence: How
Radioimmunoassays (RIA): What
Immunohistochemistry (IHC): What
Serum (antibodies, none), antigens added (bind, free), complement added (bind, unbound), sensitised RBC added (no surplus complement, binds RBC), intact RBC pellet or red cell lyse
Relies on specific antibodies binding target antigens, specific wavelength light allows visualisation of antigen-antibody complexes as fluorophore emits light, indirect fluorescence antibody test kits for T. gondii and N. caninum antibodies
Quantifies amount of specific antigen in serum, antigens labelled with radioisotopes
Detect specific antigens in formalin-fixed paraffin embedded tissue sections suing antibodies, to classify tumours
Molecular diagnostics:
Applications
Sourcing material
Polymerase chain reaction/ PCR (4)
Quantitative PCR/ QPCR
Reverse transcriptase PCT/ RT-PCR
Microarrays
Infectious diseases, genetics/hereditary diseases
DNA (double helix more secure, hydrogen bonds between base pairs, Adenine/Thymine, Guanine/Cytosine) or RNA, from blood, faeces, nasal/ ear swabs
Selective amplification of particular gene, nuclei acids detected, use of thermal cycler, amount and presence of product visualised using staining and gel electrophoresis
-Denaturation (high temp 94’C, breaks H binds, revealing bases in specific order)
-Annealing (cooler temp 50-60’C, complementary base pairing, oligonucleotide primers anneal to gene of interest, one for sense strand one for antisense strand)
-Extension/elongation (higher temp 74’C, synthesis of more complementary DNA from 3’ end of primer, only regions with primers amplify)
-Cycling (repeated multiple times for product numbers increase exponentially)
Image every cycle, detects and measures/monitors fluorescence (at primer) using thermocycler and fluorimeter, Cq value (top fluorescence) depends on relative target abundance (how much in source material to start)
Reverse-transcriptase enzyme to produce double stranded DNA from RNA, template for normal PCR, eg blue tongue
DNA extracted and broken, microarray contains many DNA probes, specific genes bind to probe and fluoresce, fluorescence read and translates to genotype, tests many in one go, eg Lawrence Livermore Microbial Detection Array LLMDA (detects presence and strains of all microbes)