Hematology Week 12 flashcards
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
74 Terms
introduction to white cell disorders
Opportunity for white cells to mobilize occurs during
Infection
Inflammation
Chronic disease
Parasitic infestations
Cell line increase is designated by “osis” or “philia”
Cell line decrease is designated by “penia”
Conditions with increased neutrophils
Infections
Inflammatory response
Stress response
Malignancies
Chemical assault
Conditions with increased eosinophils
(Normal: 0% to 4%)
Allergies
Skin disease
Parasitic disease
Transplant rejection
Myeloproliferative disorders
conditions with increased basophils
Myeloproliferative disorders
Hypersensitivity reactions
Ulcerative colitis
Conditions with increased monocytes
(Normal: 2% to 9%)
Chronic infections
Malignancies
Leukemias with a strong monocytic component
Bone marrow failure
conditions with increased lymphocytes
(Normal: 20% to 40%)
Normal in children 4 months to 6 years old
Viral (CMV)
Leukemias
Mononucleosis
Leukocytosis
refers to an increase in the total # of WBCs due to any cause
left shift
BM is responding to the increased WBC count by sending out younger cells
Bands and occasional metamyelocytes
Leukemoid reaction
Exaggerated response to infections and inflammation
WBC: 20 to 50 x10⁹/liter (20,000 – 50,000 cells/uL)
Immature cells but no blasts
disorders of the neutrophil
Disorders of the neutrophil predispose an individual to recurrent infections
Can be classified as:
Quantitative: neutrophil numbers altered
Qualitative: a defect in the neutrophil function
Neutrophil is there, but it’s not working properly
Both: number and function can be affected
This presents a problem with innate immunity and the host’s first line of defense
Neutrophilia
An increase in WBC’s with an increase in the percent of neutrophils
Can have:
Immediate Neutrophilia
Acute Neutrophilia
Chronic Neutrophilia
immediate neutrophilia
This is a pseudo-neutrophilia or physiologic neutrophilia
Can occur without a pathologic stimuli
Usually transient
Redistribution of neutrophils form the marginal pool to the circulating pool
Usually no change in the band:seg ratio
Examples that can induce this: exercise, stress
acute neutrophilia
This is a reactive neutrophilia
Occurs 4 – 6 hours of a pathologic stimuli
Increase of neutrophils from the storage pool to the circulating pool
The proportion of immature cells may increase: Band, Meta, maybe Myelo
Examples: bacterial infections, tissue necrosis, hemorrhage
chronic neutrophilia
Follows acute, if the stimulus for the storage pool release continues for a few days
The storage pool will be depleted and the mitotic pool will increase production
More immature cells form
Examples: chronic inflammation, neoplasms
neutropenia
A decrease in the white blood cell count with a decrease in the neutrophil count
Often associated with viral infections
Causes:
Decrease in bone marrow production
(stem cell damage)
Increase in cell loss
Severe infection, selective destroying, drug induced, radiation
Impaired release
Pseudo-neutropenia
Shift from circulating pool to marginal
Morphologic abnormalities
Toxic Granules: primary granules that stain basophilic color
Cytoplasmic Vacuoles: from neutrophils digesting phagocytized material
Dohle Bodies: aggregates of RNA
Transient, present during (some infections)
Often seen with toxic granulation
Hyper-segmented Neutrophils: more than 5 lobes
May be seen with megaloblastic anemia
auer rod
primary granules
found in myeloblasts or monoblasts
never in lymphocytes
Pyknotic nucleus
dense and shrunken nuclei
seen in cells that are to die and septic conditions
old blood, or severe infection
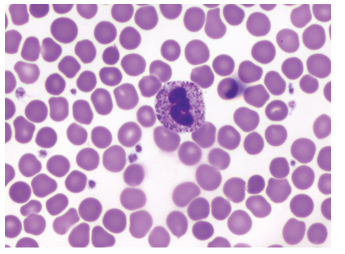
toxic granulation
Excessive amount and intensity in granulation
Response to enhanced lysosome enzyme production
Much more vivid blue-black coloration
Clusters of toxic granules appear in neutrophils
Sometimes as heavy as basophils
toxic vacuolization
Changes in segmented neutrophils
Vacuoles appear in the cytoplasm
Prolonged exposure to drugs such as sulfonamides
Blood held in storage for long periods of time: pseudo-vacuolization
Large vacuoles usually sign of serious infection and possible sepsis
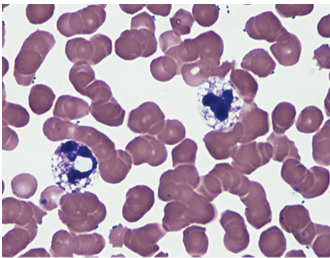
Dohle bodies
Cytoplasmic inclusion composed of ribosomal RNA
Near the cytoplasmic membrane, 1 to 5 micrometers in size
Rod-shaped pale, bluish-gray structure
Seen in neutrophils, maybe in monocytes and bands
Conditions:
Pregnancy
May-Hegglin anomaly
Bacterial infections
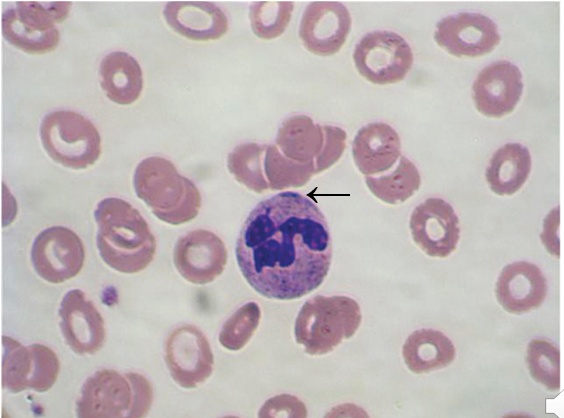
Pelger-Huet
Benign inherited autosomal dominant
neutrophil nucleus does not segment beyond the bilobed stage
Peanut or dumbbell shapes
Heavy chromatin clumping distinguishes from bands
70 – 90% of neutrophils affected with hyposegmentation
The cell functions normal
Still a mature cell, but a nuclear maturation defect
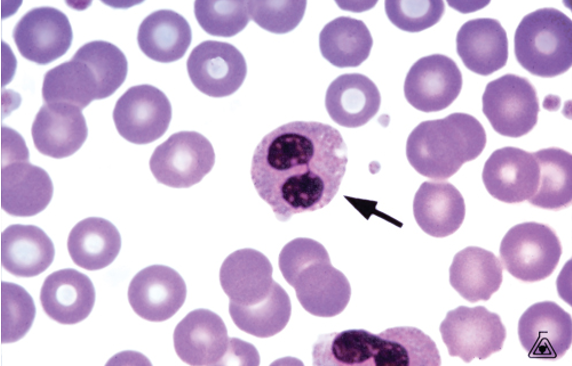
Pseudo-Pelger Huet
This is an acquired form
Found in Myeloproliferative, Myelodysplastic Syndrome and Leukemia
A maturation abnormality
Nucleus more of a round shape then that of a dumbbell
Human Ehrlichiosis
Tick Borne Disease
Present in neutrophils or monocytes
HE: Human Granulocytic Ehrlichiosis
Sometimes called HGA (human granulocytic anaplasmosis)
Anaplasma phagocytophilum
Intracytoplasmic bacterial aggregates
HME: Human monocytic ehrlichiosis
Ehrlichia chaffeensis (Rickettsia-like bacteria)
Symptoms of Ehrlichiosis
Symptoms: onset of high fever, chills, and headache, low WBC, ↑liver enzymes, thrombocytopenia
Mulberry-like inclusions may be seen in bone marrow granulocytes or monocytes
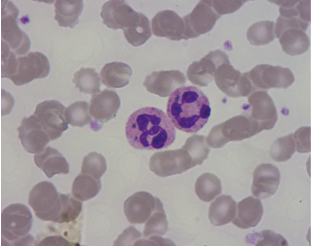
Alder Reilly
Sometimes called Alder’s anomaly
Autosomal recessive inherited disorder
Large purple granules
These granules may be found in lymphocytes and monocytes
Granules don’t affect function
Accumulation of mucopolysaccharide degradation
Enzyme deficiency
Associated with Hurlers and Hunters Syndrome
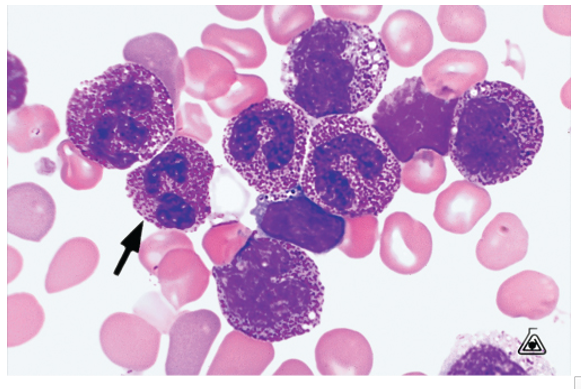
May - Hegglin
Autosomal dominant
Neutrophils, lymphocytes, and monocytes contain basophilic inclusions (>5) Dohle like
Characteristic are giant platelets
Thrombocytopenia and leukopenia
Increased susceptibility to infection
Bleeding tendencies
Decreased platelets = more bleeding
Decreased WBC = more infections
Chediak-Higashi
Rare autosomal recessive disorder
Presence of giant gray – green cytoplasmic granules in the neutrophil
The neutrophil locomotion is impaired decreasing the cell activity in killing microorganisms
Neutrophils cannot kill the infectious organisms
The first line of defense is corrupted
Significantly affects the host organism to lose that capability
Death usually occurs in infancy or early childhood due to overwhelming infections
Chediak-Higashi symtpoms
Patients tend to have albinism
Patients tend to have silver hair
Skin lacks myelin
Platelets have storage pool defects and bleeding tendencies
Impaired chemotaxis
Chemotaxis: movement of cells in a direction of a gradient.
Immune cells aren’t “called” into action
Staphylococcus aureus accounts for about 70% of infections
Chediak-Higashi syndrome
Lymphs and monos may show a single red granule in the cytoplasm
Abnormal bleeding time
Develop recurring infections with Staphylococcus aureus
↓ WBC chemotaxis and bactericidal killing function
Hepatosplenomegaly and liver failure may develop
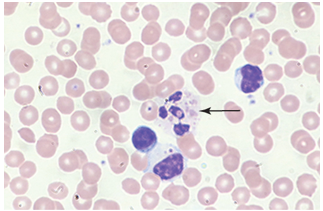
Chronic Granulomatous disease CGD
Neutrophil can phagocytize but can not kill
Defective enzyme activation of membrane oxidase
Children suffer from recurrent infections
Catalase positive organisms particular involved
Staphylococcus aureus
These recurrent infections affect the surrounding tissue producing granulomas
NBT: Nitro blue tetrazolium (oxidative burst)
Myeloperoxidase deficiency
Absence of myeloperoxidase in the neutrophil used for killing bacteria
Tends to be mild because other enzymes are more efficient
Leukocyte adherence
Absence of the leukocyte cell surface adhesion proteins (CD11/CD18)
Neutrophils fail to migrate to inflammatory sites
Flow cytometry useful for diagnosis this disorder
Eosinophilia
Increased eosinophil count
Parasitic infections
Allergic disorders
Infections such as TB, Leprosy, and fungal
Dermatitis
Malignancies: Hodgkin’s lymphoma, CML, Leukemia
Eosinopenia
Decreased Eosinophil count
Induced by stress reactions
Cushing’s syndrome
Steroid use
Sometimes caused by burns or acute infections
Basophilia
Increased basophil count
Associated with immediate hypersensitivity reactions
Frequently associated with long term foreign (antigenic) stimulation
May be seen with Myeloproliferative disorder, CML, and PV
Hypothyroidism
Basopenia
Decreased basophil count
Difficult to detect because levels are so low normally
May be a response to thyrotoxicosis
Some infections
Some acute hypersensitivity reactions
Monocyte disorders
Play important role in inflammation and immune reactions
Monocyte count increases 3 – 5 days
“cleans up” after the neutrophil
More of a tissue cell then a circulatory cell
No storage of monocytes
Not usually seen in great numbers on peripheral blood smear
Increased monocyte count during bloodborne infections
macrophage disorders
Macrophages dispose of unwanted materials
These are a group of disorders where there is a deficiency of an enzyme
Enzymes responsible for metabolic breakdown of lipids
As a result of the enzyme deficiency undigested substance accumulates within the macrophage
Largely affects Jewish or Ashkenazi Jews whose origin is from the Baltic Sea
Gaucher’s disease
Autosomal recessive trait
Can not be determined on peripheral blood
Deficiency of beta gylcosidsase
Accumulation of glycollipids within the cell
Enlarged spleen and liver
Bone marrow may be involved
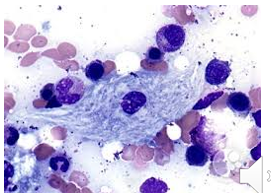
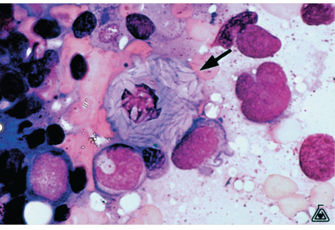
Gaucher’s disease characteristic
The diagnostic cell is termed Gaucher cell
20-80 microns, small eccentric nucleus, cytoplasm rich in lipid with a crumpled tissue paper look
Not found in peripheral blood
Types of Gaucher’s disease
Three types
Type I: most common, chronic adult type
Type II: infant – 2 years of age
Type III: sub acute early childhood to teenage years
Disease when found in infants characterized by retarded growth and CNS involvement
Severity by patients age
Leukopenia, N/N anemia, thrombocytopenia, (bone fractures)
Niemann-Pick diseases
Rare autosomal recessive
Deficiency of the enzyme sphingomyelinase
Accumulation of unmetabolized lipid, sphingomyelin and cholesterol
Macrophages can be found in the liver, spleen, lung, and bone marrow
Accumulation of sphingomyelin leads to nerve cell degeneration and growth retardation
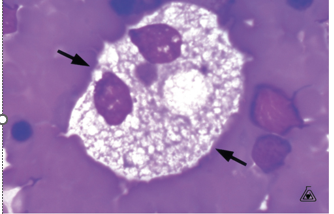
Niemann - Pick disease characteristic
Called a Foam Cell or Pick Cell
These are lipid-laden macrophages that contain cholesterol
Large 20 – 30 microns
Eccentric nucleus
Globular cytoplasmic inclusions
Niemann - Pick (cont.)
Usually fatal within the first years of life
Macrophages contain cytoplasm that is also filled with lipid droplets (mono’s also)
Type A: Infant
Type B: Chronic
Type C: Adult
Tay-Sachs disease
Autosomal recessive
Lysosomal storage metabolic disorder
Deficiency of beta – hexosaminidase A enzyme
mutation in HexA gene
HexA gene responsible for production hexosaminidase enzyme
Accumulation of gangliosides, glycolipids, and mucopolysaccharides – builds up to toxic levels and affects nerve function
Macrophages have large accumulation of lipids in attempt to remove lipid debris from ruptured neurons
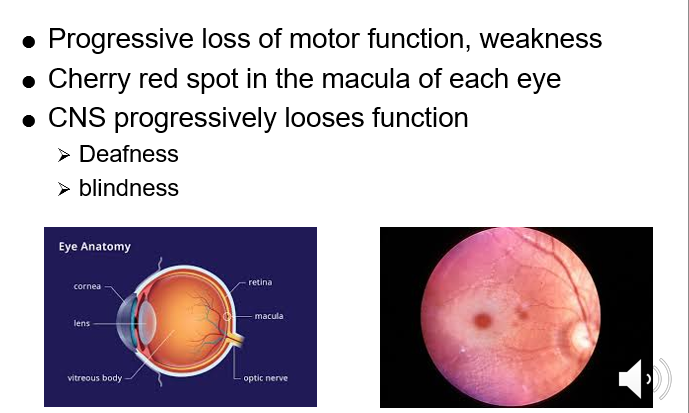
Neurodegeneration CNS system and eyes affected
Onset of symptoms course in the first few months of life
Infants fail to develop and the disease is fatal by age 4
Tay-Sachs disease characteristics
Vacuolated lymphocytes in peripheral blood and bone marrow
Spleen, liver, and lymph nodes not enlarged
Sea- Blue histocyte
Extremely rare autosomal recessive trait
Also known as inherited lipemic splenomegaly
May be associated with other disorders such as Niemann-Pick
No specific deficiency has been identified
Characterized by enlarged spleen and elevated triglycerides
Thrombocytopenia
Bone marrow aspirate needed to confirm
Lymph nodes not involved
More benign
Some labs call it a syndrome, but often it’s just a microscopy finding
Sea- Blue histiocyte characteristics
Histiocytes filled with lipid rich granules that stain blue – green with Wright Giemsa
20-60 microns
Eccentric nucleus
Cytoplasm contains granules that stain blue green
Normal lymphocyte values
Normal Adult Values: ~ 20-40%
Normal Child:
Birth: 26 – 36%
2 – 6 month: 40 – 70%
1 – 6 years: 45 – 75%
6 – 16 years: 25 – 60%
lymphocytic disorders
Can be acquired or congenital
T or B lymphocytes can be affected
Acquired disorders
Quantitative in nature (amount of lymphocytes are abnormal)
Occur as a self limited reactive process
No treatment necessary
Morphology is not normal
Reactive lymphocytes
Infectious mononucleosis
Predominance in young adult : 14 – 24 years old
Caused by Epstein Barr Virus (EBV)
Acquired disease
Transmission: oral through saliva (most common)
Relative and Absolute lymphocytosis (abnormally elevated)
See >50% lymphocytes
Positive Mono Spot / Heterophile antibody
Mono Spot can be false negative because B cells are infected
Because B cells are what produce antibodies
B cell lymphocytes are the principle target for EBV
T cells can be infected, but B cell is primary target CD21 on B cells is major receptor for EBV
IM clinical features
IM lab evaluation
Peripheral blood
Absolute leukocytosis due to lymphocytosis
12-25 x103/uL
Usually lasts 2-8 weeks
Lymphocytes >50% of leukocyte differential
>20% reactive lymphocytes
Platelets often mildly ↓
Similar morphology in other viral diseases that exhibit reactive lymphocytosis
Must carefully differentiate EBV infected cells
Toxoplasmosis
intracellular protozoan: toxoplasma gondii
one of the most common parasites worldwide
Acquired or congenital
Congenital: capable of crossing placenta
Extremely dangerous for fetus
Leads to miscarriage / still birth
If child is born with congenital infection – may not show symptoms right away, but eventually develops vision loss, mental disability and seizure
Lab results of toxoplasmosis
Leukocytosis
Relative and absolute lymphocytosis
Heterophile Titer is negative (monospot negative)
Diagnosis is established by confirmation of toxoplasma antibodies
Hematologic complication: hemolytic anemia
Cytomegalovirus (CMV)
Herpes group virus
Transmitted by: oral, respiratory, sexual contact, and blood transfusions
Resembles Infectious Mono (IM) except there is NO enlarged lymph nodes or tonsillitis
Hematologic findings in neonates: thrombocytopenia and hemolytic anemia
Incubation
Adult: 35 - 40 days
Children 20 - 25 days
Most are subclinical, CMV more common in adults
Significant if present in blood transfusion
Host may already be in weakened state or immunocompromised, which can be fatal if they receive CMV pos blood.
acquired cmv
Spread by close contact and blood transfusions
Found in urine, oral, cervical secretions and semen
Disease occurs in the immunocompromised individual
congenital CMV
Virus crosses the placenta and infects the fetus
Newborns demonstrate jaundice and enlarged liver
CMV laboratory findings
Leukocytosis with absolute lymphocytosis
Reactive lymphocytes
Heterophile Titer negative
Diagnosis is made by demonstrating the presence of IgM antibodies to CMV
A four fold rise in the IgG antibody titer is considered diagnostic
Molecular tests (DNA) also used for diagnosis
acquired lymphocytic disorders
reactive lymphocytosis
bodetella pertussis (whooping cough)
lymphocytic leukemoid reaction
Lymphocytic leukemoid reaction
plasmacytosis
Reactive lymphocytosis
Previously called infectious lymphocytosis
Reactive immune response
Associated with common viruses that affect children
Adenovirus or coxsackie virus
Leukocytosis and lymphocytosis occur first week
Small, normal, not reactive lymphocytes
Lymphocytic leukemoid reaction
Increased relative lymphocyte count with the presence of reactive or immature-appearing lymphocytes
May resemble CLL
Pertussis
Caused by Bordetella pertussis
Whooping cough
Clinical picture
May resemble infectious lymphocytosis
Leukocytosis ~ 15 - 25x103/ul (can be up to 50x103/uL)
Small, mature lymphocytes with condensed chromatin
Laboratory diagnosis
Culture, serology, P C R, immunophenotyping
Gold standard is culture; specific, not sensitive
Serologic diagnosis is based on demonstrating antipertussis toxin antibodies
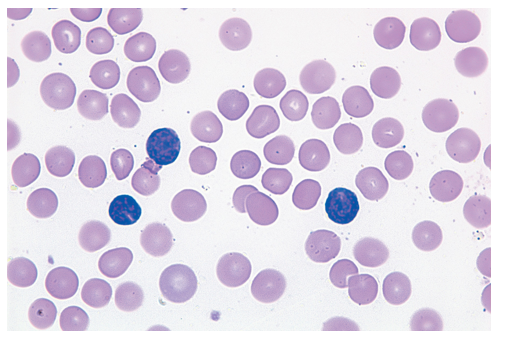
plasmacytosis
Plasma cells not normally in Peripheral Blood
Occasionally seen with intense stimulation of immune system as occurs in some
Viral, bacterial infections
Skin diseases, liver cirrhosis, collagen disorders, sarcoidosis, multiple myeloma
Morphologic variation of reactive plasma cells is flame cell (reddish purple cytoplasm)
Contain more I g than normal plasma cells
Associated with I g A plasma cell myeloma, other immune pathologies
Congenital disorders
Can affect T or B cell lymphocytes
Characterized by ↓ lymphocytes and impairment in either cell-mediated immunity (T lymphocytes), humoral immunity (B lymphocytes), or both
Severely compromises the immune system of the host
Functional immune impairment
Often apparent from birth or very young age when children have repeated infections
WBC count usually decreased
Lymphocyte morphology is normal
No reactive lymphocytes seen
SCID
severe combined immunodeficiency
both T and B are deficient
lymph nodes lack plasma cell
severe immunocompromised state - “bubble boy”
Wiskott-Aldrich
inadequate T lymphocytes but B lymphocytes are normal
Digeorge
Nonfunctional thymus and parathyroid gland
Decreased T lymphocytes, but normal B lymphocytes
X-linked Agammaglobulinemia (Bruton’s)
Normal T and Decreased B (decreased maturation)
Patients often present with respiratory and skin infections
HIV
Infects T helper Lymphocytes
Binds to CD4 protein
Suppression of cell mediated and humoral immunity
Both can’t function without working T helper cells
Lab Findings:
depends on severity or progression of infection
Diagnostic criteria when HIV infection progresses to AIDS
Use flow cytometry: CD4 T cell count falls below 200 cells/mm3 then defined as AIDS
Peripheral smear for AIDS (advanced state)
Absolute number of lymphocytes correlates with stage of disease and prognosis
Lymphopenia, leukopenia and thrombocytopenia with advanced disease
Rarely, patient may develop aplastic anemia (acquired)
The effect of HIV/AIDS on hematology parameters
HIV is the causative agent of AIDS
Lymphocytes are primarily involved in this disease
Lymphocytes show reactive changes such as extremely basophilic cytoplasm or possibly clefting and vacuolization
Normal ratio of CD4 (helper) to CD8 (suppressor) cells is 2:1, but reduction in CD4 causes ratio to be reversed and leads to a decline in immune capabilities

Laboratory evaluation of AIDS
Laboratory evaluation
Multiple hematologic abnormalities
Leukopenia
Lymphocytopenia (includes reactive forms)
May have neutropenia
Mild to moderate anemia
MCV >100 femtoLitre after receiving zidovudine
Thrombocytopenia
Low serum Fe, TIBC, ↑ ferritin