CHM 1045 mandatory memorizations
1/54
Earn XP
Description and Tags
CHM 1045 fall 2024 professor zollo
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
deci
10 -1
centi
10-2
milli
10-3
m
micro
10-6
nano
10-9
n
deca
101
da
hecto
102
h
kilo
103
k
mega
106
M
giga
109
G
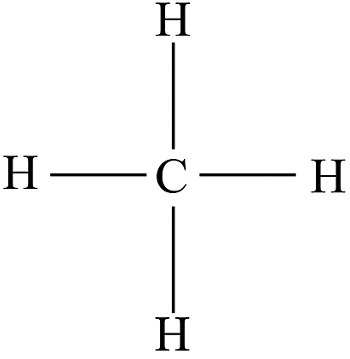
what is the prefix for this?
meth

what is the prefix for this
eth
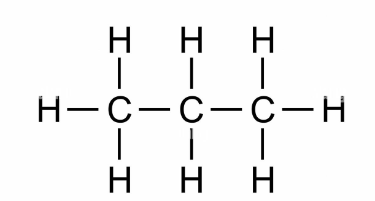
what is the prefix for this
prop
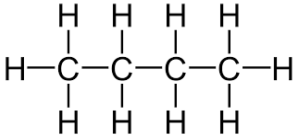
what is the prefix for this
but
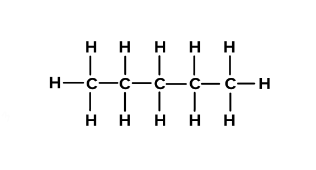
what is the prefix for this?
pent
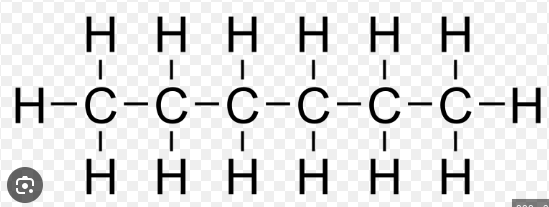
what is the prefix for this?
hex
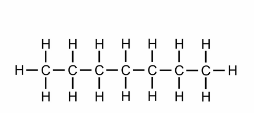
what is the prefix for this?
hept
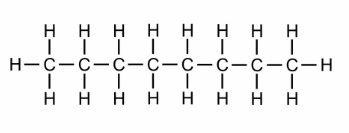
what is the prefix for this?
oct
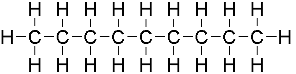
what is the prefix for this?
non

what is the prefix for this?
dec
what do hydrocarbon prefixes end with?
-ane
mono
1
di
2
tri
3
tetra
4
penta
5
hexa
6
hepta
7
octa
8
nona
9
deca
10
c = λν
speed of light formula
c
3×108 m/s
speed of light
λ
wavelength
v
frequency
h
6.626×10-34 Js
planck’s constant
n principal quantum number
shows electron energy level
l
shows the shape (s,p,d,f), 0 to n-1
Ml quantum number
describes the orientation of the electrons in the orbital and the number of different orbitals -1 to 1
spin
electron spin direction: clockwise +1/2, counterclockwise -1/2
compound
substances with 2 or more elements
ionic compounds
a metal and a nonmetal
electrostatic attraction
holds opposites together (cations and anions)
what suffer do you use with ionic and covalent compounds ?
-ide
which type of compound uses a number prefix?
covalent
Fe has a change of…
2+, 3+
Ni has a charge of…
2+, 3+
Cu has a charge of…
+, 2+
Zn has a charge of…
2+
Hg has a charge of…
Hg2+ Hg2+2
Sn has a charge of…
2+, 4+
Pb has a charge of…
2+, 4+
polyatomic ions become an acid with the addition of a _____ atom
hydrogen
when an oxygen is taken away from a polyatomic ion what happens?
it ends with “-ite”
when an oxygen is taken away from a polyatomic ion but the charge is positive what happens?
it ends with “-ous”