chemistry atoms elements and compounds
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
____ ___ was the first scientist to develop atomic theory
John Dalton

John Dalton was the first scientist to develop _____ _____
atomic theory
all _____ is made up of _____
matter, atoms
_____ within an _____ are the same
atoms, element
____ CANNOT be broken down
atoms
atoms are __________ during a ______ reaction, but are not lost (law of _____ of _________)
rearranged, chemical, conservation, mass
________ were discovered by passing electricity through a _________________
electrons, cathode ray tube
______ were discovered by shooting _______ with ______ ______
protons, gold foil, alpha particles
_______ were discovered by shooting ____________ through ________, causing protons to be ejected
neutrons, alpha particles, beryllium
atoms are made of __________
subatomic particles
what is the nucleus
dense, positively charged core of matter
protons are _____ and have a mass of _ ____ and determine the _____ of an atom
positively charged, 1 amu, identity
elements are _____ with unique ______
atoms, identities
nuetrons have _______ and a mass of _ ___ and holds the nucleus together
no charge, 1 amu
the job of the proton is to ___________
the job of the nuetrons is to ________
-determine the identity of an atom
-hold nucleus together
atomic mass is found by
adding total number of protons and neutrons
electrons have a ________ mass
negligible
______ are held within due to the attraction to ______
electrons, protons
Specifies the mass AND atomic number
nuclear notation
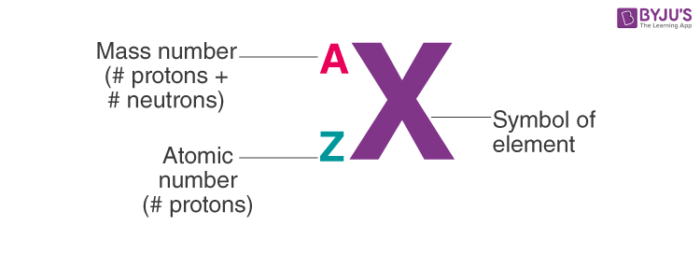
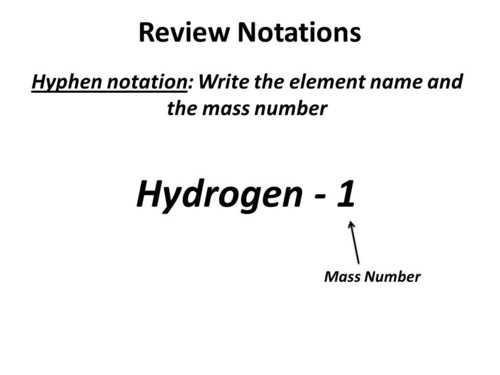
specifies mass
hyphen notation
atoms are _______ charges
neutrally
losing electrons = _____ion
positive
gaining electrons =______ion
negative
positively charged ions are called
CATIONS
negatively charged ions are called
ANIONS
isotope
atoms of the same element can have different number of neutrons
different number of _______=different mass
neutrons
elements mass on the periodic table have an _____ value because of the existence of ______
average, isotopes
what is the formula for finding the average atomic mass?
%(Mass)+%(mass)+…= __amu
the side to side rows are called
periods
how many periods are there
7
what are the up and down colomns called
groups
how many groups are there
18
metals are broadly classified as:
metals, nonmetals, metalloids
where are metals located
left of stairs
where are nonmetals located
right of stairs
where are metalloids located
6 elements near stairs
Alkali metals: very _______ _____
Halogens: very _____ ______
Noble gases: very _______
Alkali metals: very reactive metals
Halogens: very reactive nonmetals
Noble gases: very unreactive

name the Alkali metals, alkaline earth metals, inner transition metals, transition metals, halogens, Noble gasses

____ are composed of atoms held together by ______ _____
compounds, chemical, bonds
Ionic bonds occur between ______ and _______
metal, non metal
transfers electrons (metals→ nonmetals)
ionic bonds
covalent bonds occur between ________ and _____
non metal, non metal
covalent bonds form _____
molecules
in a covalent bond, electrons are _______
shared
Al3+ S2-
monoatomic
SO2- NO1-
polyatomic
Na→Na1+
monovalent
Cu→Cu2+ or Cu1+
multivalent
if the anion does NOT contain oxygen: Hydro______ic acid (HCl=hydrochloric acid)
if the anion does NOT contain oxygen: Hydro______ic acid (HCl=hydrochloric acid)
if anion DOES contain oxygen:
ends in -___→ _____ic acid
-ate
if anion DOES contain oxygen:
ends in -___→ ous acid
ite
what are the types of chemical formulas?
empirical, molecular, structeral
emperical:
smallest whole number ratio of atoms in a compound

molecular:
actual number of atoms in a compound
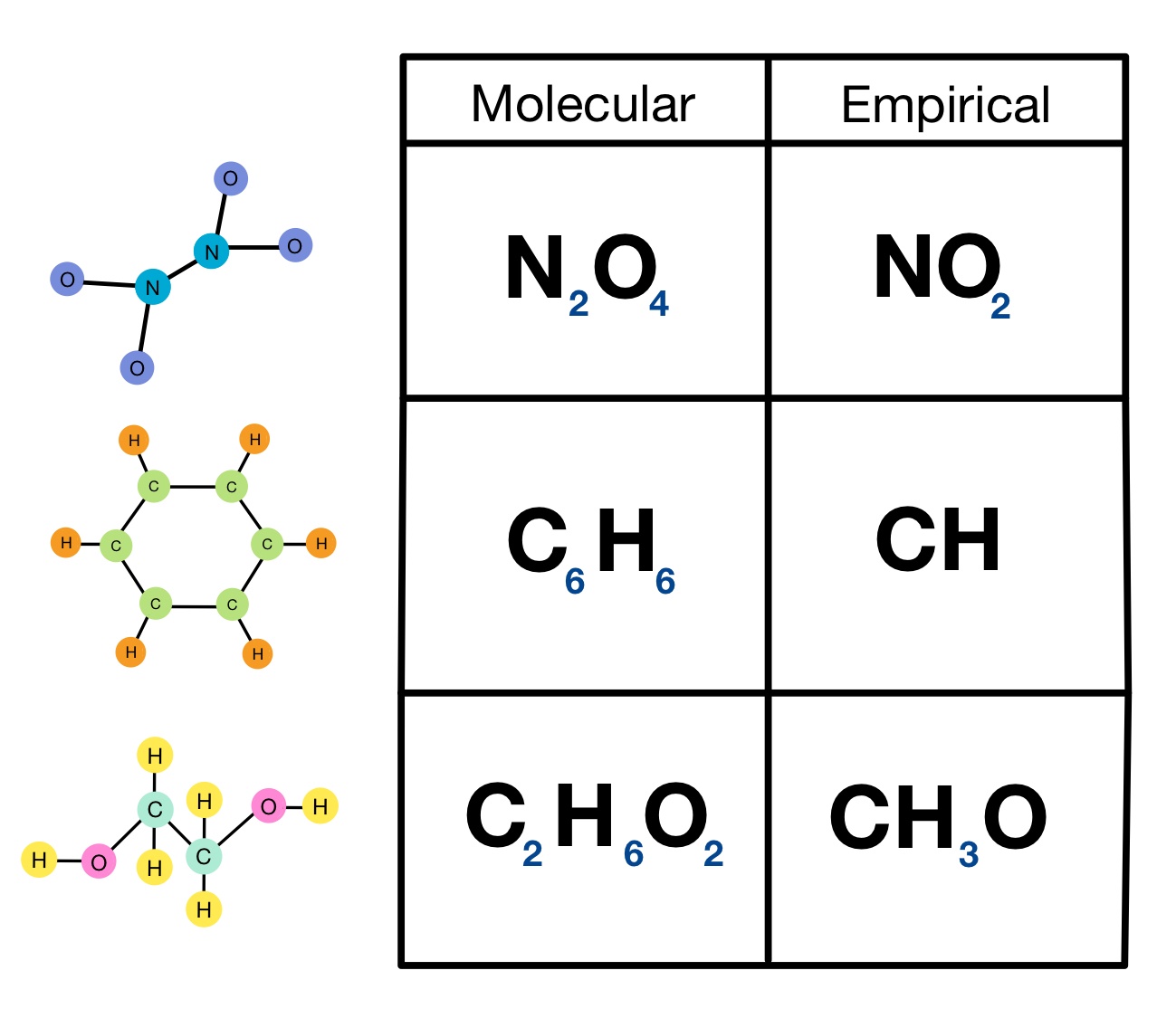
structural
uses lines to represent covalent bonds and shows how atoms bond to each other