Topic 3 - Particle Model of Matter
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is the formula for density?
Density = Mass / Volume
What are some properties of a solid?
Strong forces of attraction
Fixed, Regular arrangement
Vibrate on a fixed position
High density
What are some properties of a liquid?
Weaker forces of attraction
Particles are close and can move past eachother
Irregular Arrangement
More energy
Random
Less dense
What are some properties of a gas?
Almost no forces of attraction
Lots of energy
Free to move
Random but fast
Low density
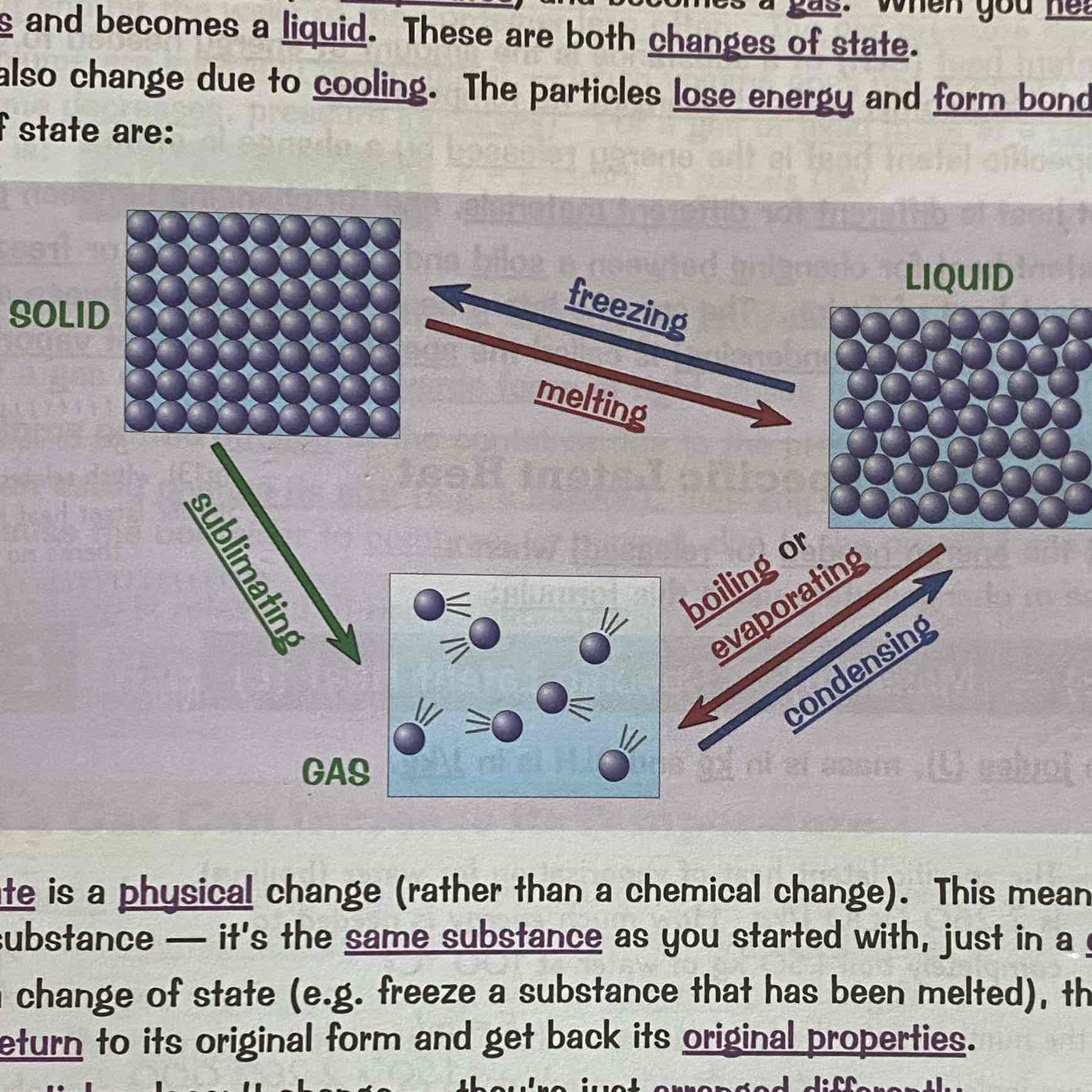
What are the changes of state?
See image:
What is specific latent heat and formula?
The energy needed for a 1kg mass to change state without changing its temperature.
E = ML
E = Energy
M = Mass
L = Specific Latent Heat
What is the specific latent heat called for solids, liquids and gases
Solid Liquid - Specific latent hear of fusion
Gas - Specific latent heat of vaporisation
How does temperature affect kinetic energy?
The higher the temperature, the higher the kinetic energy store. So the higher the temperature of the gas, the higher the speed of the particles.
How can pressure be increased?
There needs to be more collision for a higher pressure
Faster particles
Increased temperature
How does volume affect pressure?
Volume is inversely proportional to pressure.
How can pressure change volume?
If there is pressure outside the object and if the object can easily change shape (balloons) then a change in pressure can compress or expand the object.
How does work/force affect temperature?
If you transfer energy by applying a force then you do work. This increases the gas’ internal energy and therefore increases its temperature.