F4 rr2 physics transfer of thermal energy
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
describe one experiment to demonstrate the properties of good thermal conduction involving tiles.
comparing conduction in tiles: stand with bare feet or hands. one food should be placed in tiles/stones and the other on a rug. it will feel as though the tile is colder however they are the same temp. the difference is that the tiles are good thermal conductors so where the foot touches the tiles heat is transfered away from the foot, making it feel colder as it loses heat to the tiles. on the other hand, textiles such as rugs are good insulators, meaning they are poor heat conductors. this means on this foot heat is not transfered therefore it doesnt change temp and it will feel relatively warmer compared to the other foot which lost heat to the tiles.
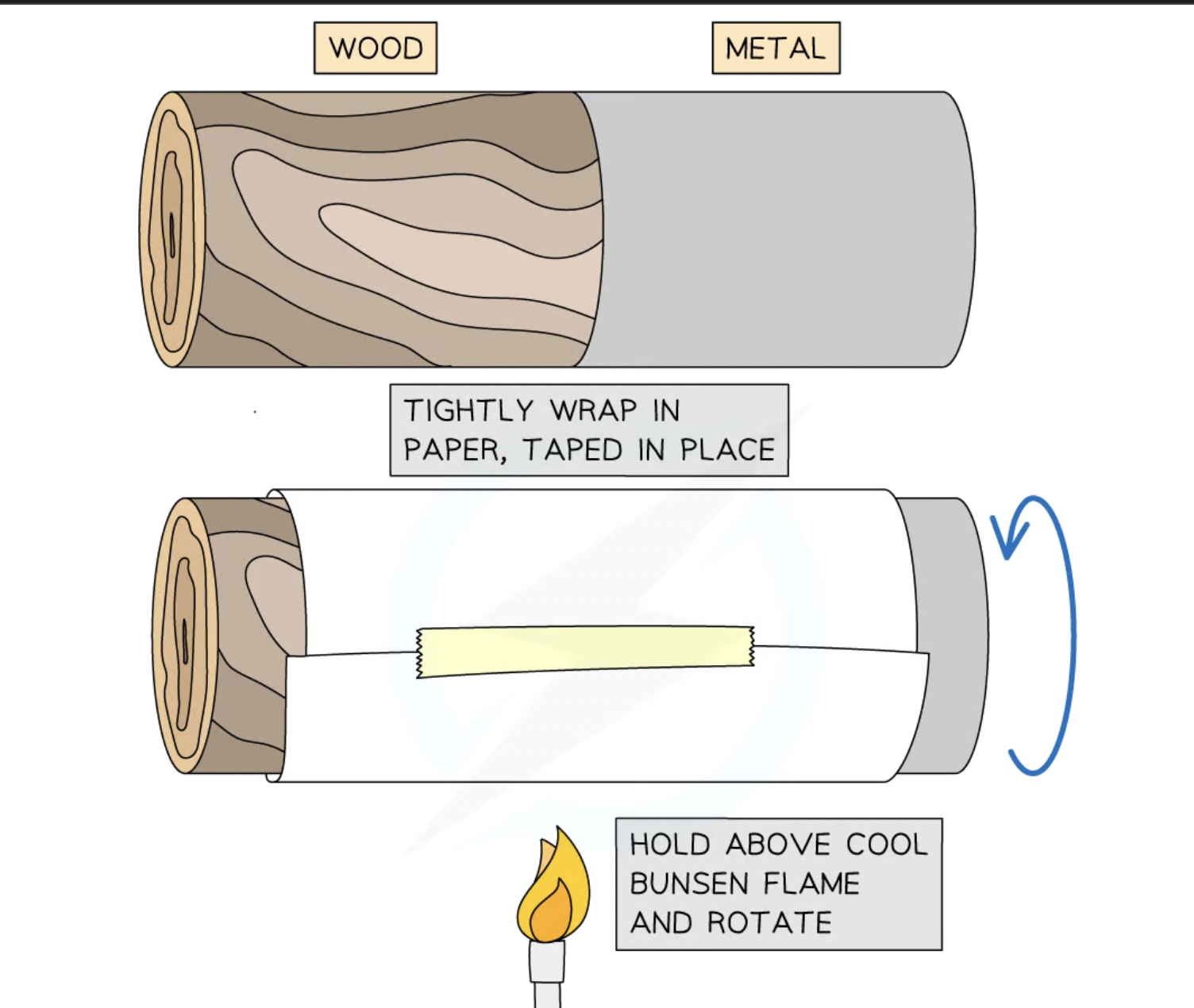
describe an experiment to demonstrate the properties of good thermal conduction with the use of wood and paper
rod .using a gentle flame, gently heat the paper at the join of the wood and metal. keep turning the rod so the paper is well heated all around the circumfrence. stop when the paper is clearly discoloured. unroll the paper and observe the distinct pattern: where the paper touched the metal surface it is undamaged whereas where the paper touched the wood it is charred. this is because metal is a good heat conductor so heat was transferred from the paper into the metal. however wood is a good insulator therefore heat was not transferred from the paper and burnt.
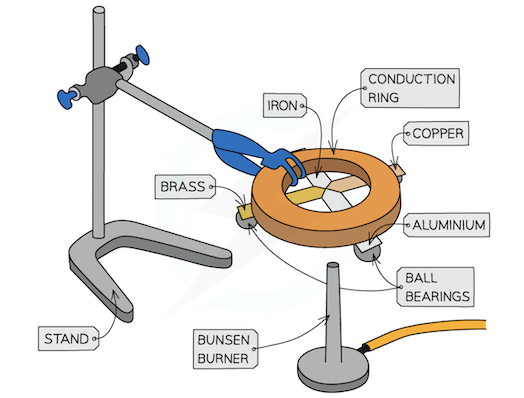
describe an experiment to demonstrate the properties of good thermal conduction by demonstrating different rates of thermal conduction in metals
ball bearing should be stuch to each of the metal strips using wax and then turned upside down. the strips should be be gently and equally heated using a bunsen so each strip is strip needs to be heated at the point where they meet. when the heat reaches the ball bearings the wax will melt leading the ball which was previously stuck to fall. by timing how long it takes for each strip, their relative thermal conductivity can be determined.
what is conduction?
conduction is the transfer of heat from one region to another through particle vibration and the movement of free electrons.
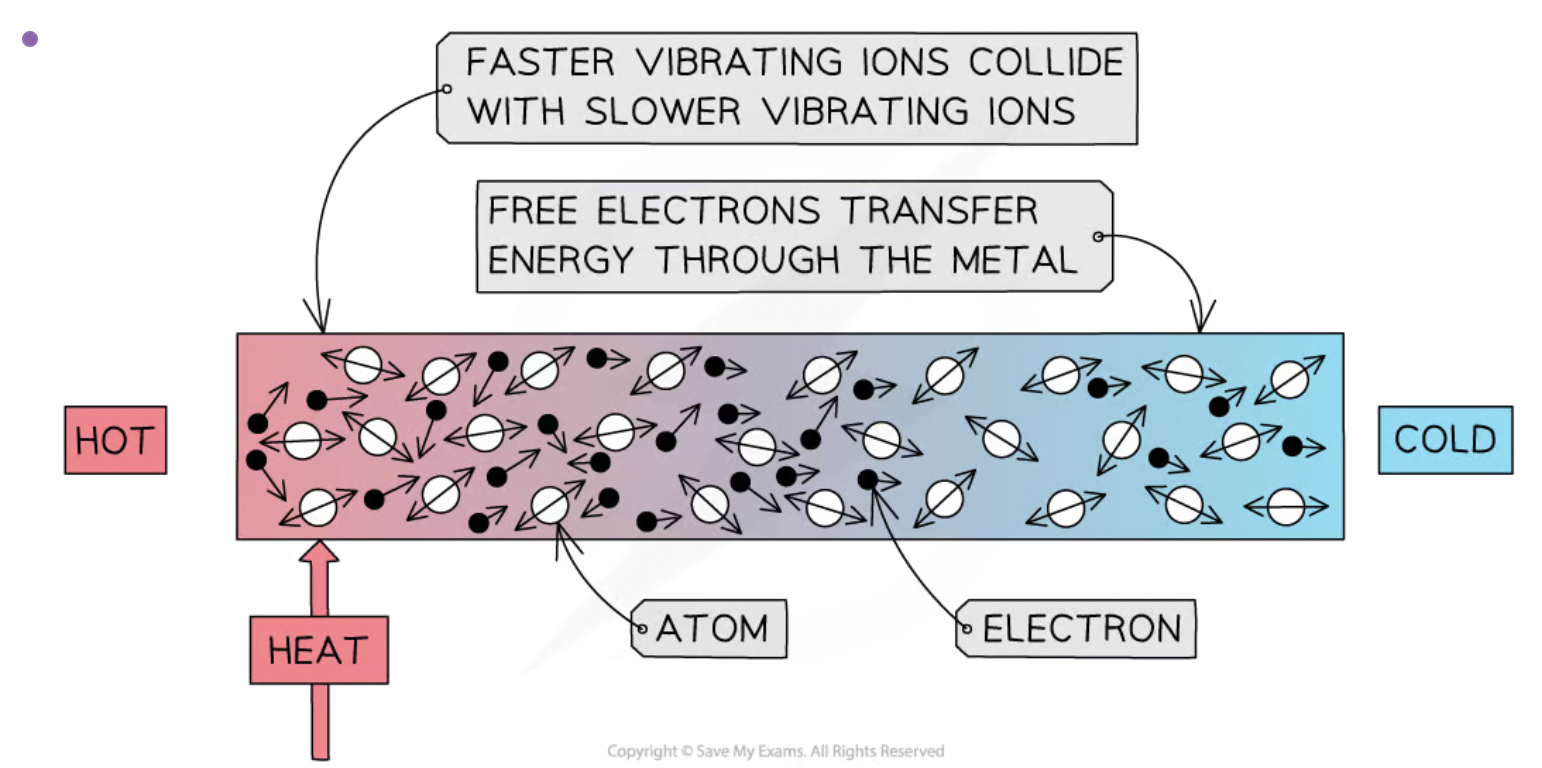
describe thermal conduction in all solids in terms of atomic or molecular lattice vibrations and also in terms of the movement of delocalised electrons in metallic conductors
conduction can occur through 2 mechanisms: atomic vibration and free electron collision.
when a substance is heated, the atoms or ions start to vibrate. as the atoms at the hotter end vibrate they bump into the atoms at the colder end transfering energy from atom to atom. these collisions transfer internal energy until thermal equilibrium is achieved through out the substance.
describe in terms of particles why thermal conduction is bad in gases and most liquids
for conduction to happen, the particles need to be close together so that when they vibrate the vibration is passed along. this does not happen easily with fluids as in liquids particles are close but slide past each other, and in gases particles are widely spread appart and will not nudge eachother if they vibrate. in addition both types of fluids are poor heat conductors
list and explain which solids conduct thermal energy better than thermal insulators but less effectively than good thermal conductors
conductors tend to be metals. better thermal conductors are those with delocalised electrons which can easily transfer energy. these include: (in order from better conductors to better insulators) copper iron carbon water air glass rubber.
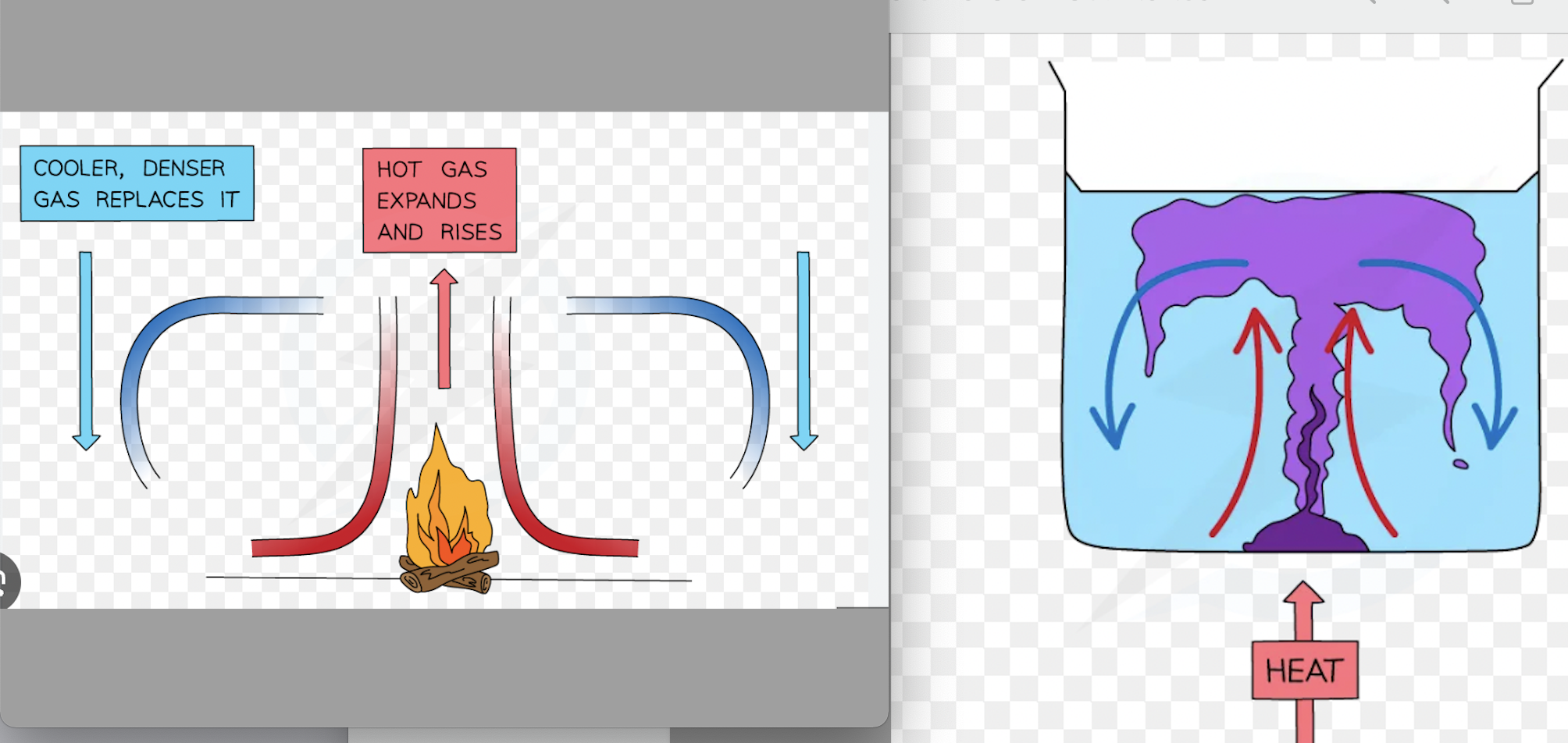
explain convection in fluids in terms of density changes
when a fluid is heated, its molecules vibrate and push each other appart, making the fluids expand and therefore turning it less dense. The hot liquid/gas then rises and the cooler molecules (more dense) move down and occupy its space. eventually, the hot fluid cools and contracts, increasing back its density, and sinking back down again where the process is most likely to repeat. this is called a convection current
describe experiments that may trial convection in liquids and gases.
explain radiation.
all objects give off thermal radiation, the hotter the object is, the more thermal radiation it emmits. thermal radiation is infrared radiation. it is the only way heat can travel through a vaccum and it does not need a medium to travel unlike convection and conduction.
describe the effect of surface color and texture on the emission, absorbtion and reflection fo infrared ratiation
Dark, rough surfaces are best for absorbing and emitting IR. Light, smooth surfaces are best for reflecting IR.
how does the rate in which an object transfers or recieves energy affects it temperature?
as an object absorbs thermal radiation it will become hotter, as it gets hotter it will also emit more thermal radiation. eventually, the object will reach a point where it is absorbing at the same rate that it is emmiting radiation. an object to be at constant temperature in needs to transfer energy at the same rate in which it recieves energy. at thermal equillibrium an object has a constant temperature.
what happens to an object if the rate in which it recieves energy is more than the rate at which it transfers.
if the rate at which an object recieves energy is greater than the rate it transfers away the objects temperature will increase.
describe an experiment to distinguish between good and bad emmiters of infrared radiation.
To distinguish between good and bad emitters of infrared radiation, an experiment can be conducted using two identical cans—one black and one shiny—filled with the same amount of hot water. After measuring the initial temperature of the water in both cans, they are placed in a controlled environment away from sunlight. Over a set period, temperature readings are taken at regular intervals to observe how quickly each can cools down. The expectation is that the black can, being a better emitter of infrared radiation, will lose heat more rapidly than the shiny can, which reflects radiation. This experiment illustrates that darker surfaces emit infrared radiation more effectively than shiny surfaces.
describe an experiment to distinguish between good and bad absorbers of infrared radiation
two identical metal cans—one matte black and one shiny silver—are filled with equal amounts of hot water. Thermometers measure their initial temperatures before placing both cans at an equal distance from an infrared lamp. The temperature of the water in each can is recorded at regular intervals for 5 minutes. The results show that the black can absorbs more infrared radiation, causing the water to heat up faster, while the shiny can reflects more radiation and heats up more slowly. This demonstrates that black surfaces are good absorbers of infrared radiation, whereas shiny surfaces are poor absorbers.
describe how the rate of emision and radiation depends on the surface area and surface temperature of an object
Surface Area: Objects with a larger surface area emit radiation at a higher rate because more of their surface is exposed to the surroundings. This is why animals in hot climates often have large ears to help lose heat more efficiently.
Surface Temperature: The hotter an object is, the more infrared radiation it emits. According to the Stefan-Boltzmann law, the rate of radiation increases rapidly as temperature rises, meaning hotter objects emit significantly more energy than cooler ones.
explain some everyday aplications and consequences of conduction
Good conductors help transfer thermal energy quickly
Examples include:
Metal pans to heat food quickly
Metal radiators to transfer heat from water inside to the surrounding air quickly
Bad conductors (insulators) help retain thermal energy as they transfer heat slowly
Examples include:
Plastic handles of saucepans to slow thermal energy transferred to hands
Air spaces in the walls or windows of some houses help to retain heat, as air is a poor conductor
explain some everyday aplications and consequences of convection
Common applications of convection are:
heating a room with a radiator
steam rising which cools a hot liquid
explain some everyday aplications and consequences of radiation
Solar Panels: Solar panels absorb infrared and visible radiation from the Sun to generate electricity or heat water.
Vacuum Flasks: The inner walls of a vacuum flask are coated with shiny surfaces to reduce heat loss by radiation, keeping liquids hot or cold for longer.
Spacecraft Temperature Control: Satellites and spacecraft use reflective coatings to control heat absorption and emission, preventing overheating from the Sun's radiation.
explain the concequences of conduction, convection and radiation when a fire is burning wood as in this case more than one type of thermal energy is significant.
In a fire, heat moves in three ways: radiation, conduction, and convection. Radiation lets heat travel through the air, warming you even if you’re not touching the fire. Conduction heats the wood or coal, helping the fire spread. Convection happens when hot gases and smoke rise, carrying heat upwards. All three work together to make the fire burn efficiently, but if there's not enough airflow, the fire can burn less effectively.
explain the concequences of conduction, convection and radiation when there is a radiator in a car as in this case more than one type of thermal energy is significant.
A car radiator cools the engine by using conduction, convection, and radiation. Conduction lets the hot coolant transfer heat to the radiator’s metal. Convection moves air through the radiator to carry away that heat. A fan helps if the car isn’t moving. Radiation also releases a bit of heat, but it’s a smaller part. If the radiator is blocked, it can’t cool the engine properly, and bigger radiators work better by giving off more heat.