BIOC*2580: Biological Molecules
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
lipids
A structurally diverse group of molecules. They are not defined by their chemical structure, but by their common chemical property: hydrophobicity (most hydrophobic of all biological molecules).
Use organic solvents to dissolve lipids (typically a 2:1 mixture of chloroform and methanol).
biological functions of lipids
Energy storage: triacylglycerols; fats and oils
Structural elements of biological membranes: phospholipids and sterols
Signal transduction (cell-cell communication): steroid hormones, prostaglandins
Enzyme cofactors: coenzyme Q (mitochondrial electron transport chain)
Vitamins: vitamins A, D, E, K
Light-absorbing pigments: carotene
glycolipids
Lipids can occur covalently linked to other classes of biomolecules.
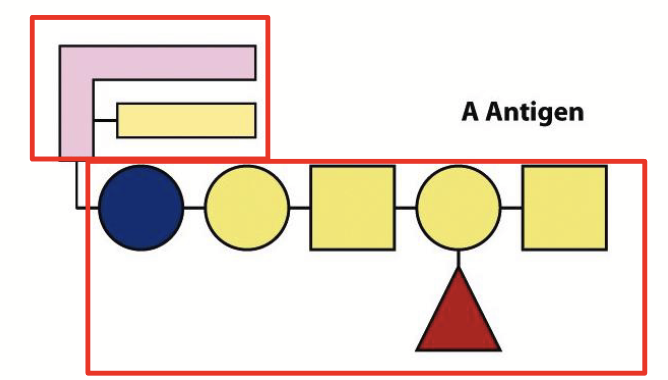
Contain both sugar and lipid portions. Important constituents of cell membranes.
e.g. sphingolipids, gangliosides
The human blood groups (O, A, B) are defined by these displayed on the outer surfaces of blood cells.
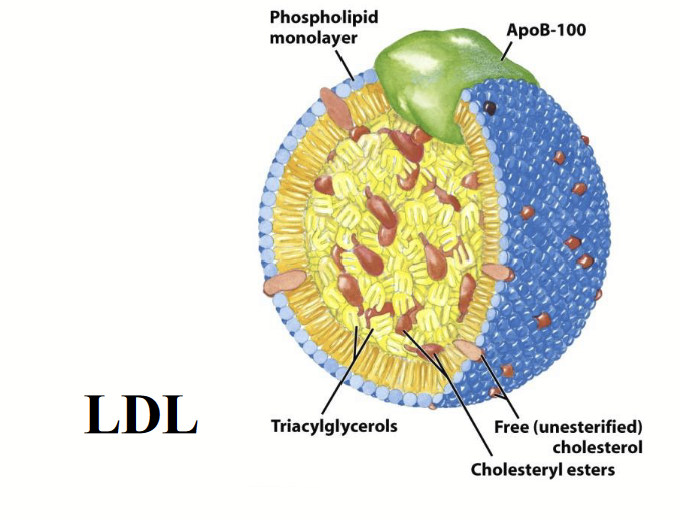
lipoproteins
Lipids can occur covalently linked to other classes of biomolecules.
Many proteins also incorporate covalently-bound lipids.
e.g. VLDL, LDL, and HDL are plasma forms (transporting lipids in the blood; lipids are hydrophobic whereas blood has water → lipids cannot just dissolve in blood to transport) that are associated with cardiovascular health and disease.
fatty acids
The simplest type of lipid. Free forms present are only found in trace quantities. Building block of many complex lipids. Central intermediates in metabolism.
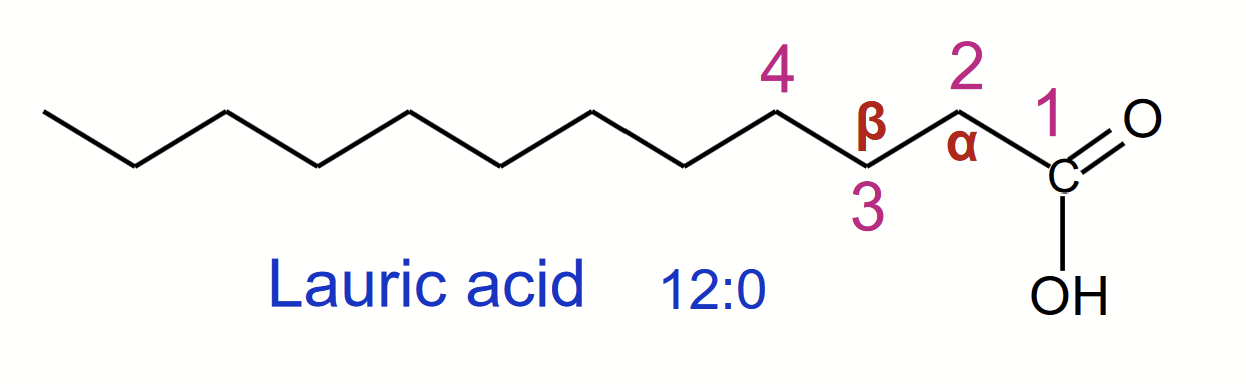
Carboxylic acids (based on pKa and pH, could be carboxylate, especially if it’s in free form) with hydrocarbon chains ranging from 4-36 carbons.
No double bonds between carbons in the chain are described as saturated.
One (monounsaturated) or more (polyunsaturated) double bonds (triple bonds are not common) in the chain are called unsaturated.
When naming, #1 is assigned to the carbonyl carbon (C-1) and the alpha to the carbon next to it.
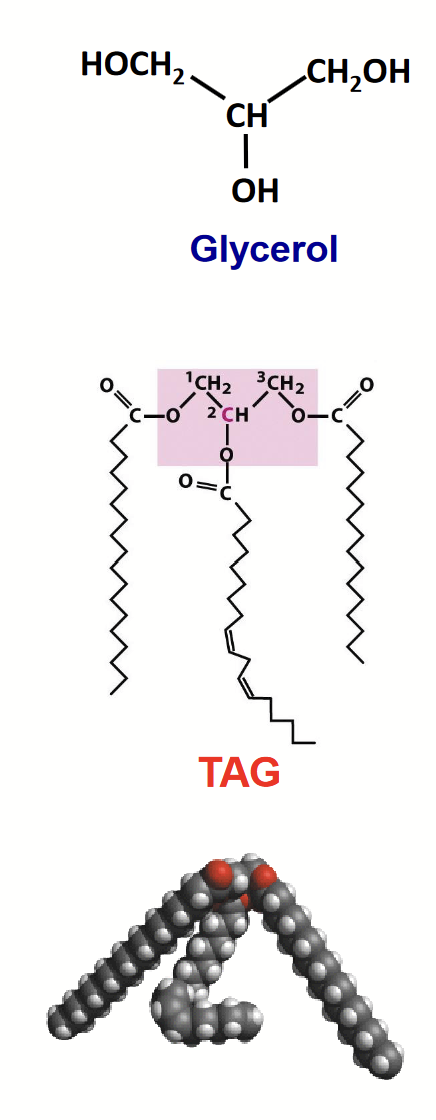
triacylglycerols
The form that the majority of fatty acids in biological systems are found. A major constituent of bulk fats and oils, including the human body’s depot fat (storage fat).
Formed by linking three fatty acids to a glycerol (alcohol) molecular through ester linkages.
Highly hydrophobic as the polar carboxylic acids of the fatty acids are tired up as (less polar) esters.
Melting points depend on the length and degree of saturation of their fatty acid constituents (higher the amount of long chain saturated fatty acids, higher the melting temperature of a natural fat).
Most natural fats are complex mixtures of simple and mixed forms. Simple: same fatty acid in all 3 positions. Mixed: 2 or 3 different fatty acids
phosphoglycerides
The major lipids in membranes.
saturated fatty acids
Fatty acids with no double bonds between carbons in the chain.
Adopt extended conformations and therefore pack in a fairly orderly way; extensive favourable interactoins.
As the chain length increases: melting point increases, solubility decreases (because more hydrocarbons are added, more nonpolar, more hydrophobicity).
Found as solids at room temperature.
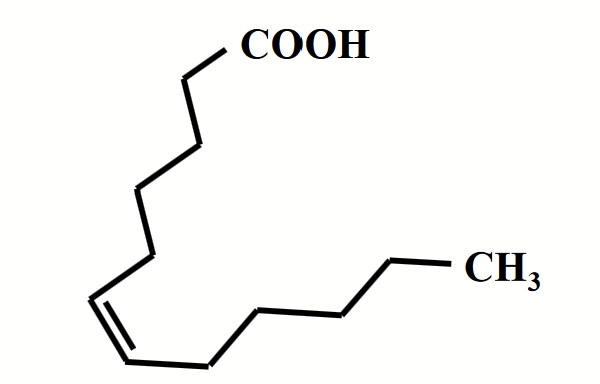
unsaturated fatty acids
Fatty acids with one (monounsaturated) or more (polyunsaturated) double bonds (triple bonds are not common) in the chain.
Pack less regularly due to the kink caused by the cis double bond, lowering the number of favourable interactions. Less thermal energy is required to disrupt disordered packing, as a result, the melting temperature is drastically lowered. The fluidity of lipid membranes depends a great deal on this fact!
Found as liquids at room temperature.
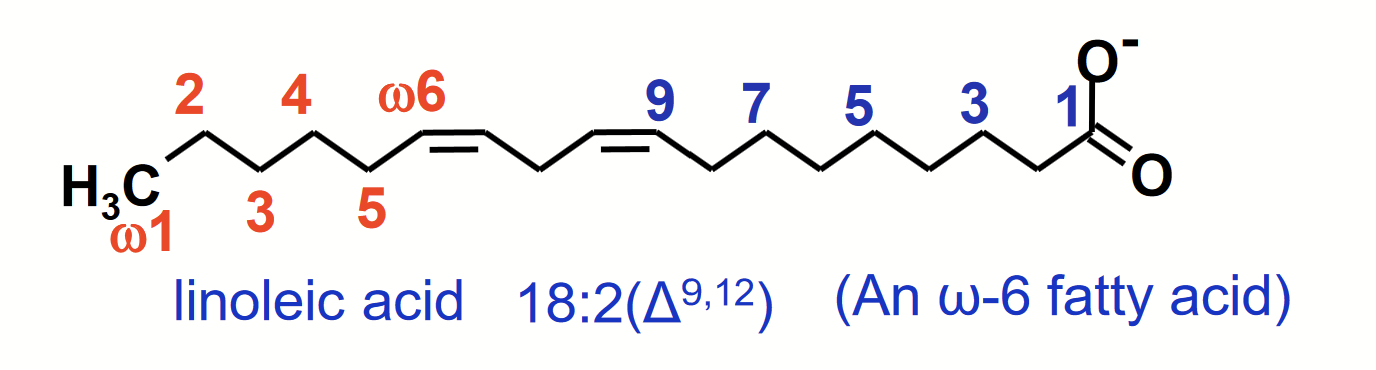
naming fatty acids
Simplified nomenclature used to describe fatty acids specifies the number of carbons in the chain followed by the number of double bonds, separated by a colon.
The position of double bond(s) in unsaturated fatty acids is specified relative to the carboxyl carbon (C-1) by superscript numbers following Δ (e.g. 18:2(Δ9, 12).
Alternative convention for polyunsaturated fatty acids is to specify the position of double bonds relative to the methyl carbon (omega, ω), commonly used to signify ω3 and ω6 fatty acids.
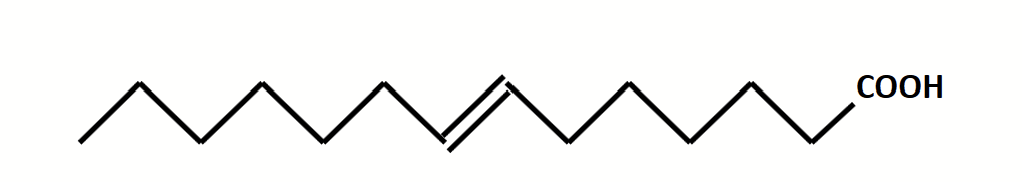
features of fatty acids
They have an even number of carbon atoms (odd numbers are very rare, though they still occur).
They are unbranched.
The double bonds are commonly found in cis configuration, which introduces a kink in the chain (not elongated chains anymore; bends).
Double bonds in PUFA are methylene-bridged, not conjugated. They are separated by a methylene carbon and therefore the bond pattern is double-single-single-double.
polyunsaturated fatty acids
PUFA
trans fatty acids
The process of partial hydrogenation of unsaturated fatty acids (converting some double bonds to single bonds), used in manufacturing margarine and similar products can isomerize the double bonds, generating this. Very few are naturally found.
A trans double bond allows a given fatty acids to adopt an extended conformation.
Due to their extended nature, can pack more regularly and show higher melting points than cis forms, allowing them to remain as a solid at room temperature (not as high of a melting point as saturated fatty acids due to the double bond).
These synthetic fats are believed to have serious negative effects on cardiovascular health.
esters
Derivatives of fatty acids (fatty acids are also carboxylic acids):
Carboxylic acids and combine with alcohols to form…
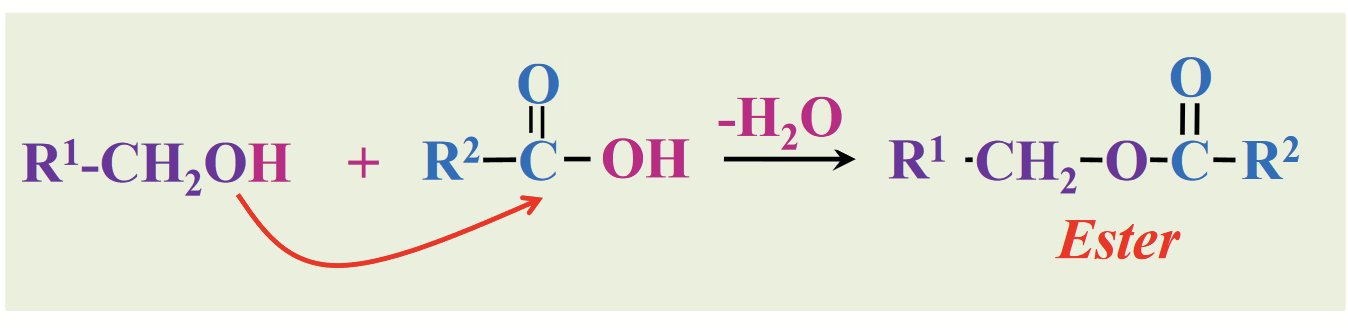
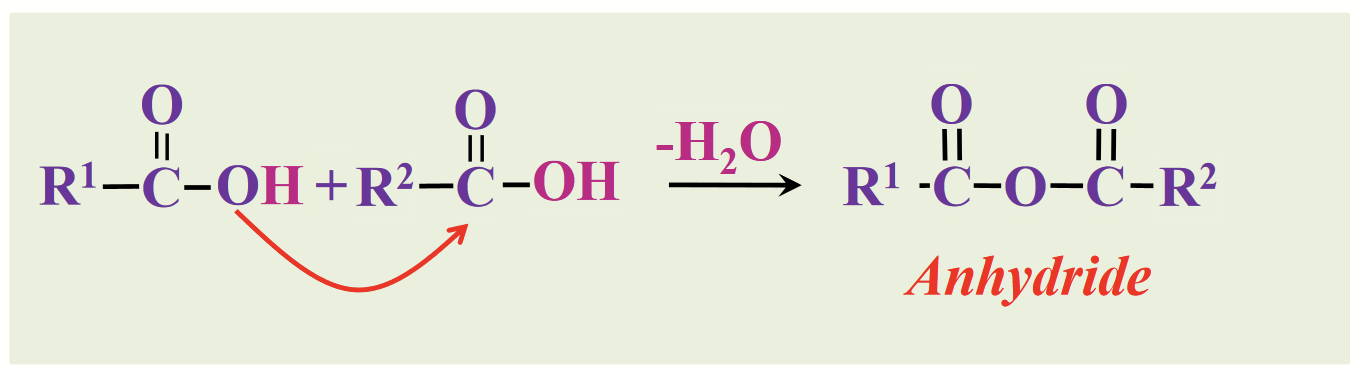
anhydrides
Derivatives of fatty acids (fatty acids are also carboxylic acids):
Carboxylic acids can combine with acids to form…
simple triacylglycerols
Triacylglycerols with the same fatty acid in all three positions.
mixed triacylglycerols
Triacylglycerols with 2 or 3 different fatty acids.
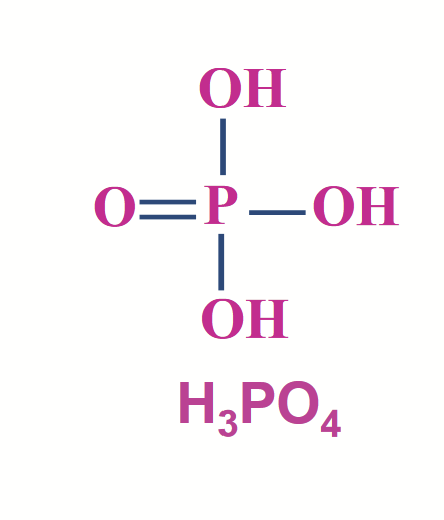
phosphoric acid (H3PO4)
Phosphate derivatives are found throughout biochemistry.
A triprotic acid (3 dissociable protons; 3 different pKa values). At neutral pH, it exists as an equilibrium mixture of H2PO4- and HPO42-. This mixture is represented by the notation “Pi”.
Essentially a carboxylic acid but the C is replaced by P - all reactions of carboxylic acids can happen to these as well. No carbons! Common: diester bond.
Pi
Notation that represents phosphoric acid at neutral pH. It exists as an equilibrium mixture of H2PO4- and HPO42-.
phosphorylation
Adds negative charges to molecules, leading to an increase in water solubility (e.g. phospholipids, DNA, RNA, and many proteins).
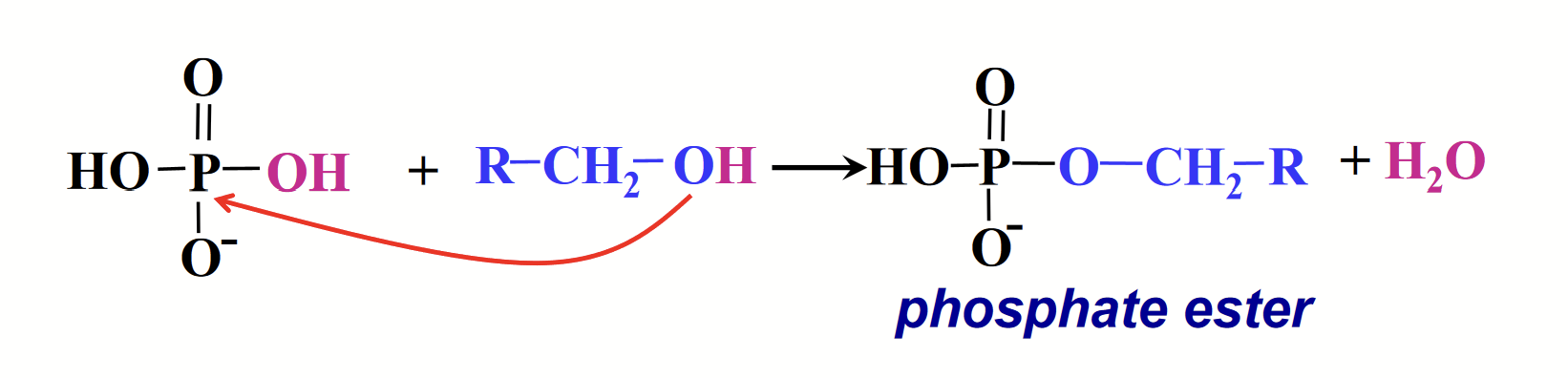
phosphate ester (phosphoester)
Phosphoric acid can react with alcohols to form…
The P in phosphoric acid makes a really good electrophile. The O in the alcohol makes a really good nucleophile once the H+ is removed.
“-POOR” group.
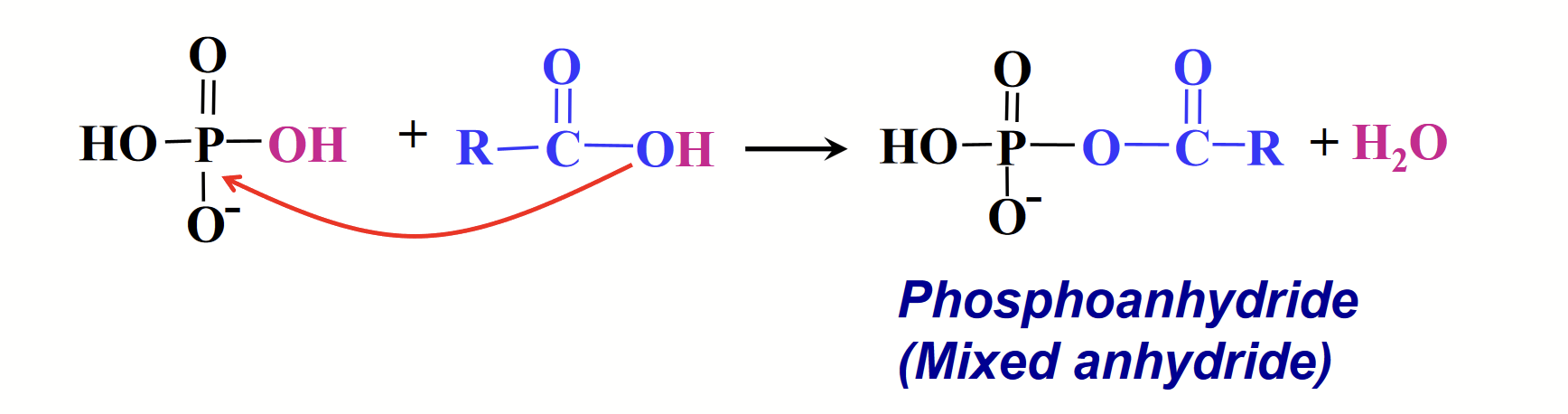
phosphoanhydride (mixed anhydride)
Phosphoric acid can react with acids to form…
The P in phosphoric acid makes a really good electrophile. The O of the “OH” in carboxylic acids makes a really good nucleophile.
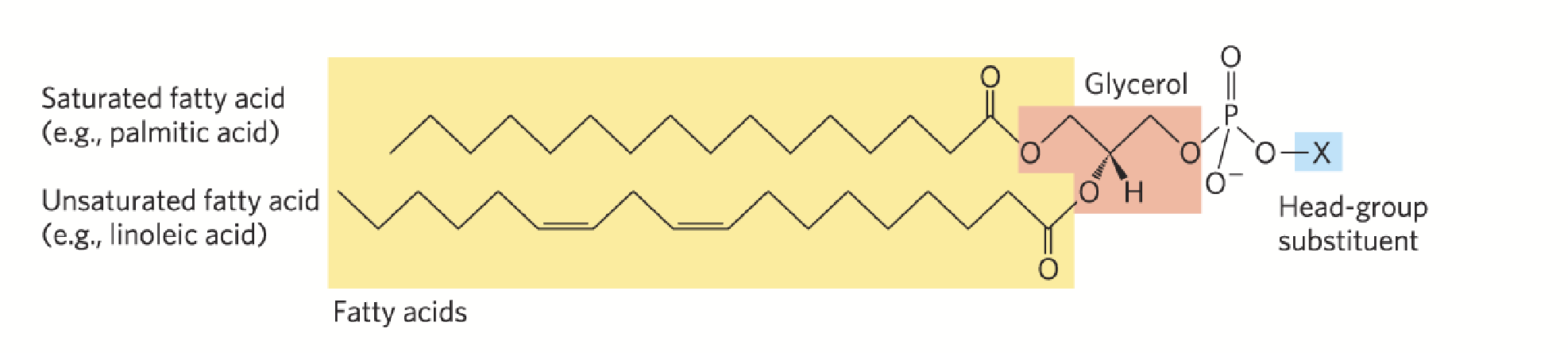
glycerophospholipids (phosphoglycerides)
Primary constituents of biological membranes.
Carbon atoms 1 and 2 of glycerol are esterified to two fatty acids (“tail”) while a highly polar or charged group (X) is attached through a phosphodiester linkage to the third (“head”).
Amphipathic - they combine both hydrophilic (head portion) and hydrophobic (tail) properties. This property differentiates them from triacylglycerides and allows them to form lipid bilayers.
phosphatidylcholine (lecithin)
Like all other glycerophospholipids, represents a class of lipid rather than a single molecule.
Different combinations of fatty acids at positions R1 and R2 correspond to different molecules.
R1 and R2 are ANY fatty acids. Structure can differ!
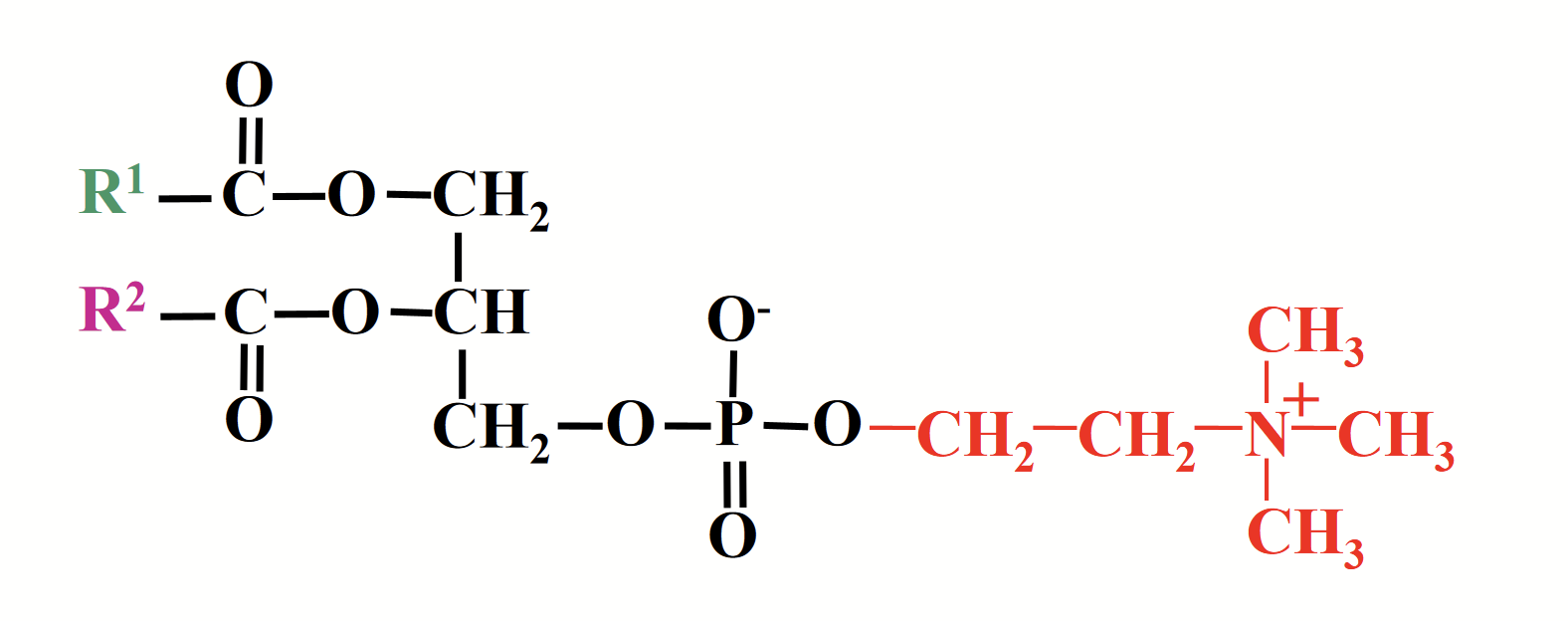
lipid aggregation
Lipids aggregate spontaneously to form complexes when dispersed in water.
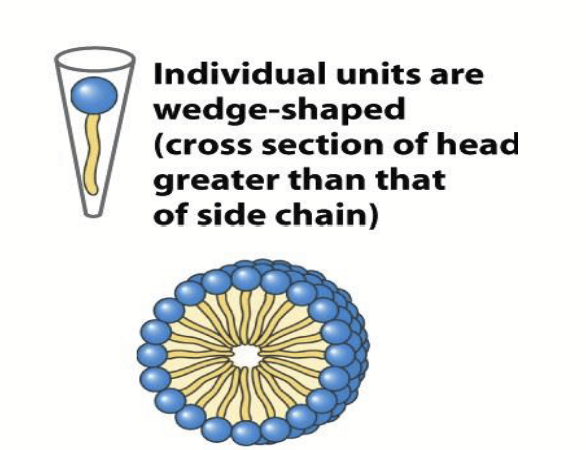
Fatty acids (and some other lipids) aggregate to form roughly sperical micelles (the smallest and simplest lipid aggregate).
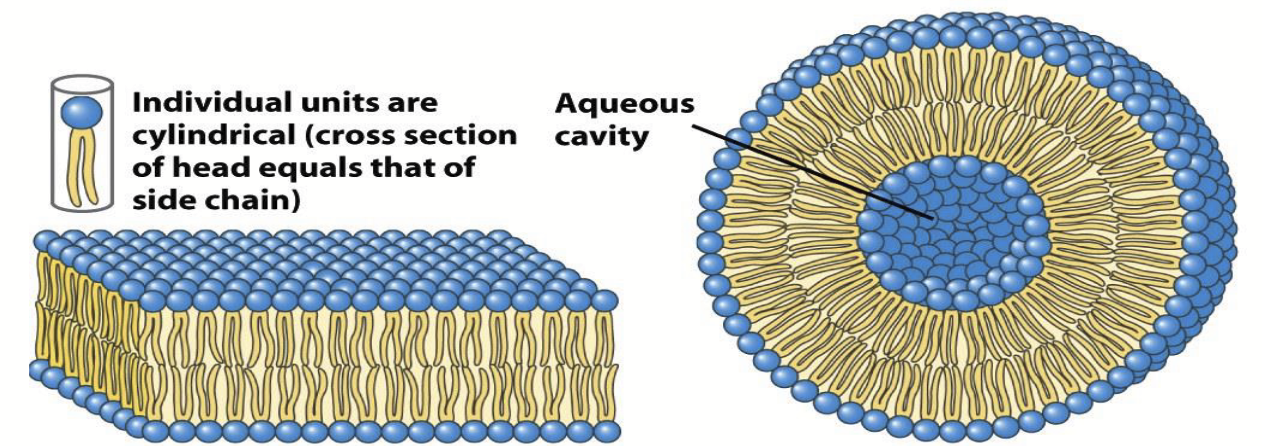
Since the hydrophobic tails of phospholipids are too bulky to pack tightly together in micelles, they aggregate into bilayers; which spontaneously fold back on themselves to form liposomes or vesicles with diameters as large as 1 micron or more (the fundamental structure of the cell membrane).
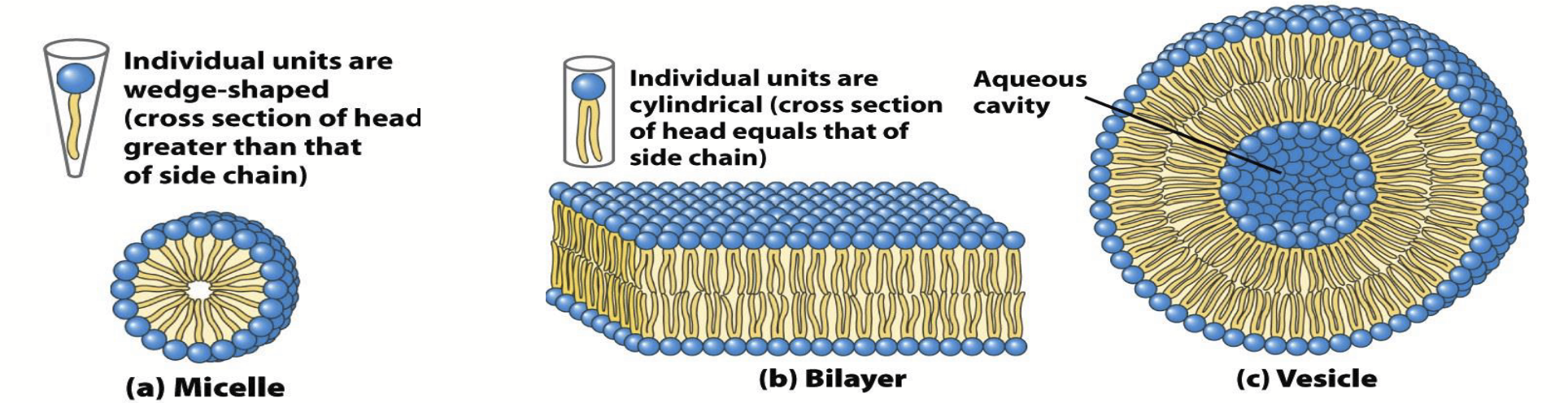
micelle
The smallest and simplest lipid aggregate; diameters of ~3-a few hundred nm.
liposomes or vesicles
Phospholipid tails (hydrophobic) are too bulky to pack tightly together in micelles, so they aggregate into bilayers, which spontaneously fold back on themselves to form these complexes.
Diameters as large as 1 micron or more.
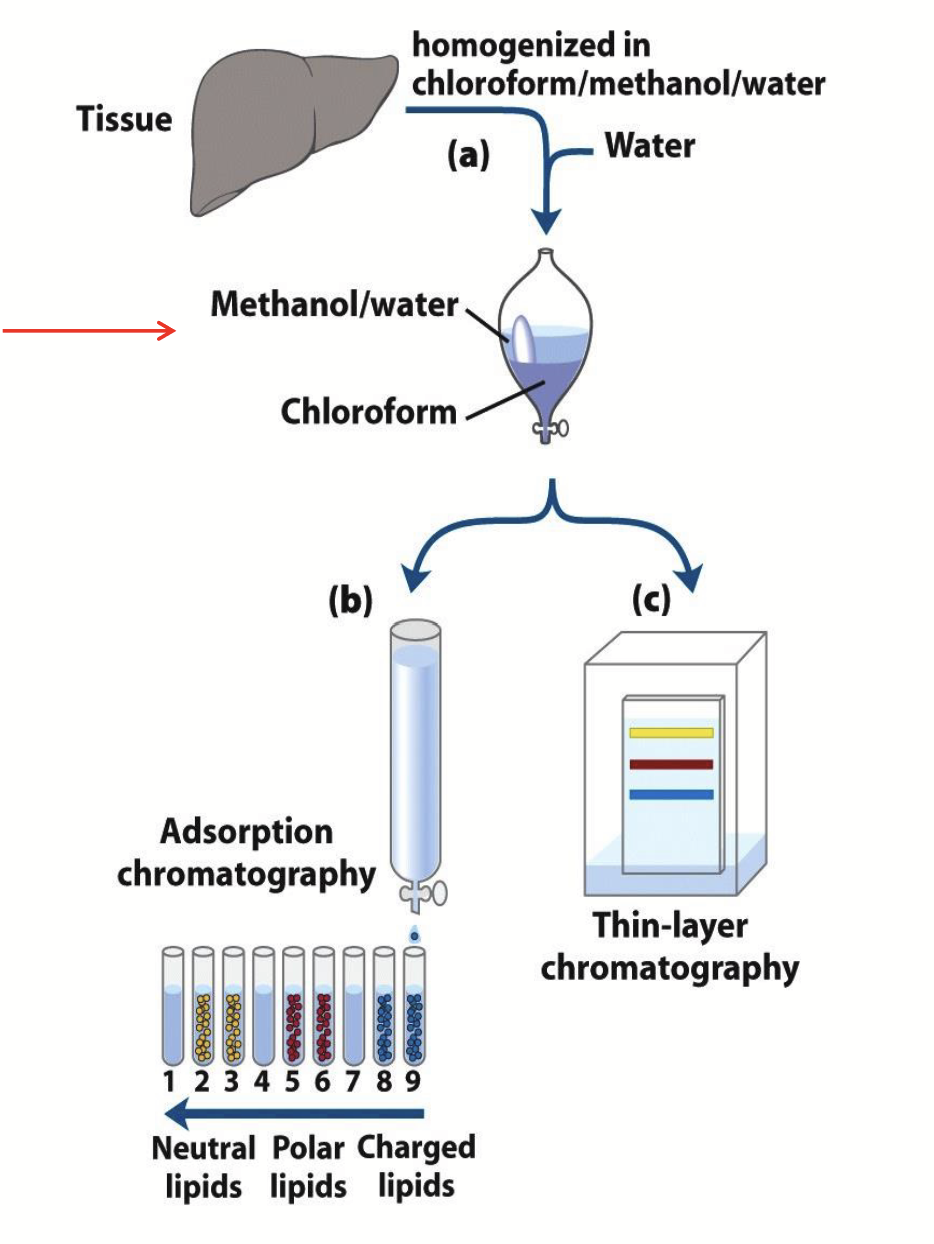
two-phase extraction (lipids)
Analysis of lipids
Separated based on their polarity on a column of silica gel or through TLC.
Progressively polar lipids elute from the column as solvents of increasing polarity are passed through it.
On the thin layer plate, less polar lipids move farther than the more polar lipids.
The methyl esters separate on the basis of chain length and degree of saturation. The separated fatty acids can be definitively identified through mass spectrometry.
carbohydrates
The most abundant biomolecule on earth.
Unlike proteins, which are built from a limited number of building blocks, the number of possible molecules is huge.
They are of great importance in energy metabolism and are essential components of nucleic acids.
monosaccharides
Simple sugars; consist of a single sugar unit (e.g. glucose).
Very water-soluble. Poorly soluble or insoluble in organic solvents such as ether or hexane. Colourless. Most are sweet to the taste.
Approximate formula: (CH2O)n
Combine two organic chemical functional groups: carbonyl (C=O) group, which is either an aldehyde or a ketone, and at least two carbons bearing hydroxyl (alcohol; -C-OH) groups.
All except dihydroxyacetone contain one or more chiral carbon atoms, giving rise to optically active isomeric forms.

oligosaccharides
Short chains of monosaccharide units.
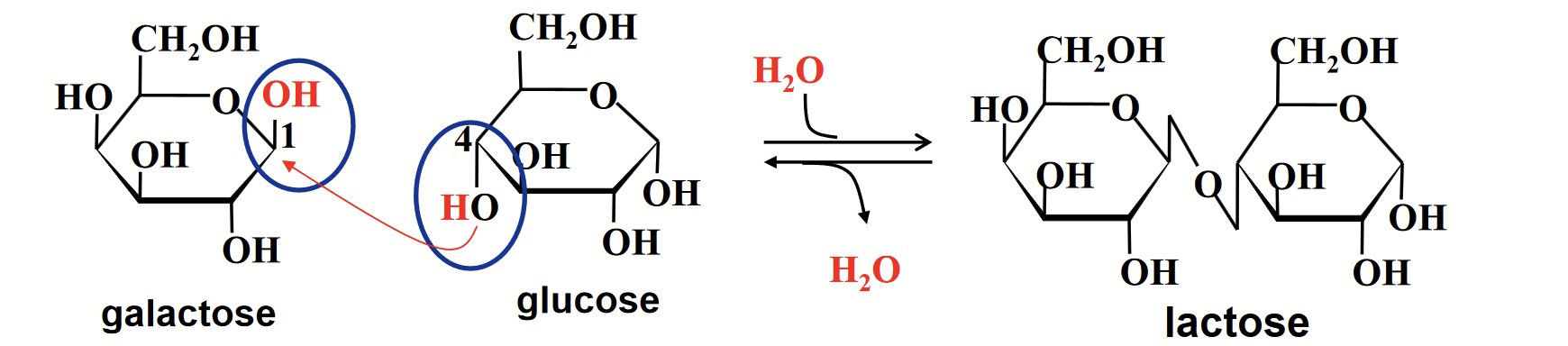
disaccharides
Consist of two monosaccharide units (e.g. sucrose).
Formed when two monosaccharides are linked through a glycosidic bond. The anomeric carbon (electrophile) of one sugar reacts with a hydroxyl group (nucleophile) of the other.
polysaccharides
Polymers of 20 or more sugar units (e.g. glycogen, cellulose).
The form that most carbohydrates in nature occur as.
Often highly branched - possible as sugars have several OH groups, each of which can act as the nucleophile in forming a glycosidic bond, bonding a single subunit to two (or more) others.
DIffer from each other in the identity of the sugar units that are linked, in the length of the chains, in the type of bonds linking the units, and in the degree of branching.
trioses
The simplest monosaccharides contain three carbon atoms: one carbonyl group and two carbons bearing hydroxy groups.
Each of these groups have aldoses and ketoses: aldotriose & ketotriose, aldotetrose & ketotetrose, etc.
Sugars with four, five, six, and seven C atoms are found in nature. The hexoses are the most common monosaccharides in nature.
glyceraldehyde (aldose)
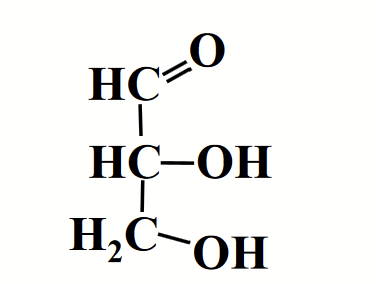
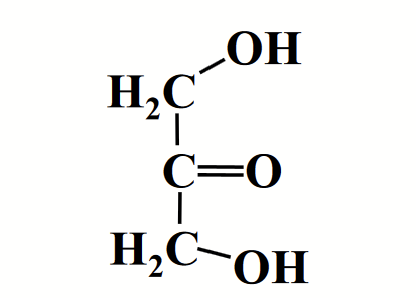
dihydroxyacetone (ketose)
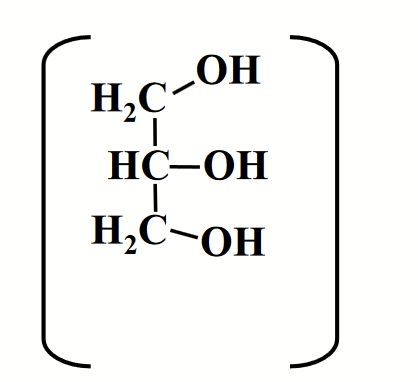
glycerol
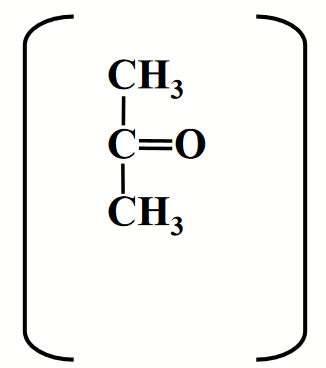
acetone
Emil Fischer (1852-1911)
Nobel Prize in Chemistry 1902.
Studied the analysis, synthesis, and stereochemistry of simple sugars, and introduced much of the terminology and notation that we still use today.

Fischer projection formula
Used to represent three-dimensional sugar structures on paper.
Vertical bonds project behind the plane.
Horizontal bonds project out of the plane.
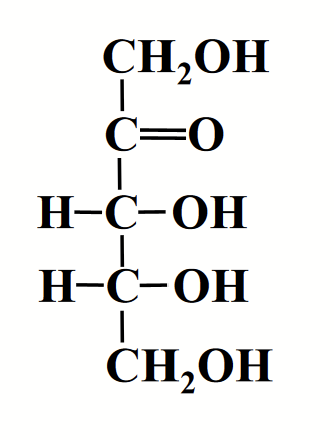
perspective formula
Method of drawing sugar structures.
Solid wedge-shaped bonds project in front.
Dashed bonds point away.
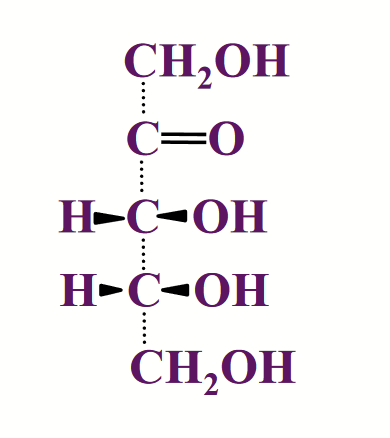
enantiomers
Mirror Images → differ in configuration at every chiral carbon atom. Commonly called “left-handed” and “right-handed” forms of a molecule.
Have identical chemical properties (e.g. melting point, water solubility).
Differ in “optical activity” → the plane of polarization of polarized light in bent in opposite directions when passing through solutions of the two.
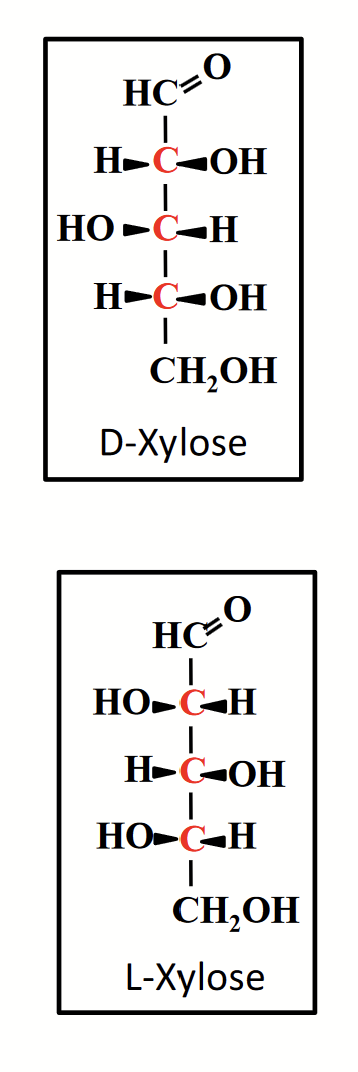
diastereomers
Monosaccharides with more than one chiral carbon atoms. Have stereoisomers that differ in handedness at some carbon atoms but not at others.
DO NOT HAVE IDENTICAL CHEMICAL PROPERTIES, because the spatial relationships among the atoms making up the molecule are different.
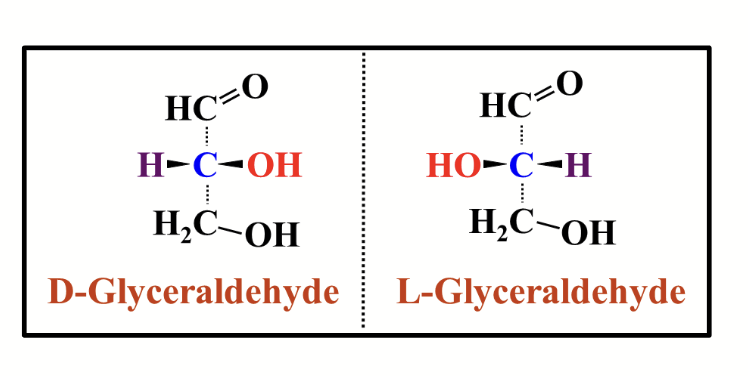
D sugar
A sugar if the chiral carbon atom furthest away from the carbonyl group has the same configuration as D-glyceraldehyde.
Makes up most (but not all) naturally occuring sugars!
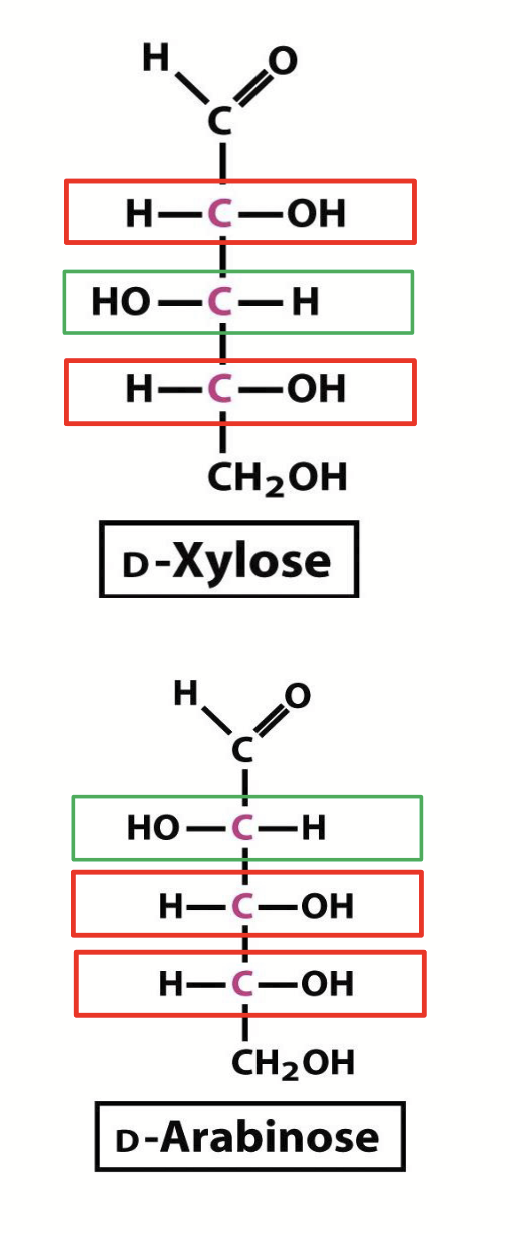
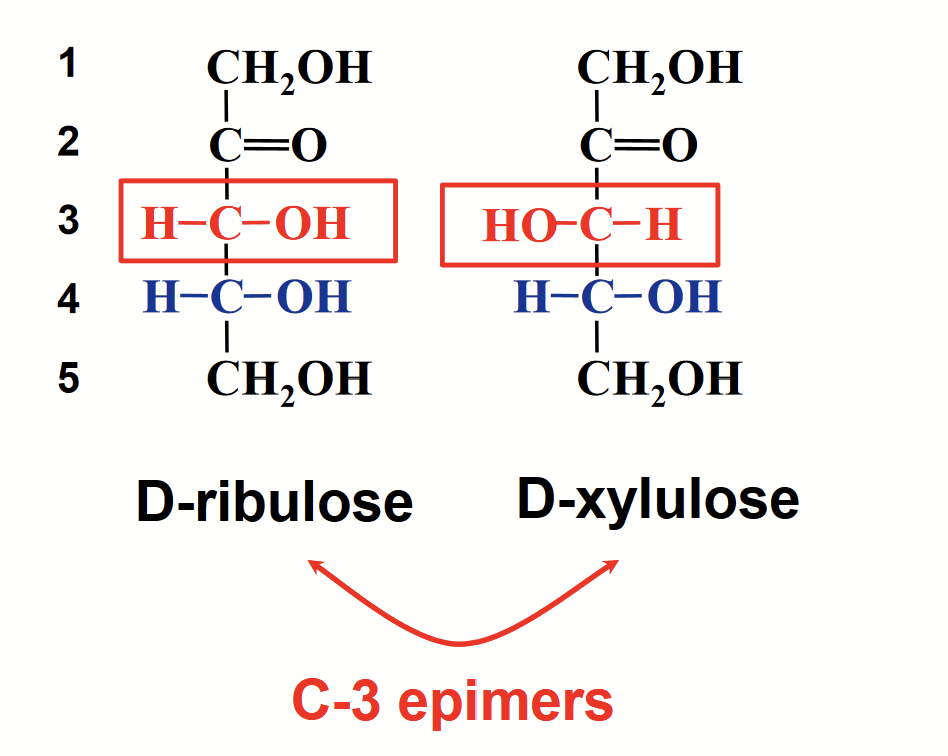
epimers
A pair of sugars that are identical except for the configuration at one carbon atom (special case of diastereomer by 1 chiral C atom).
e.g. C-3 epimers
2n
A sugar with n chiral centres has __ stereoisomers.
Half of these stereoisomers are D sugars and the other half are L sugars.
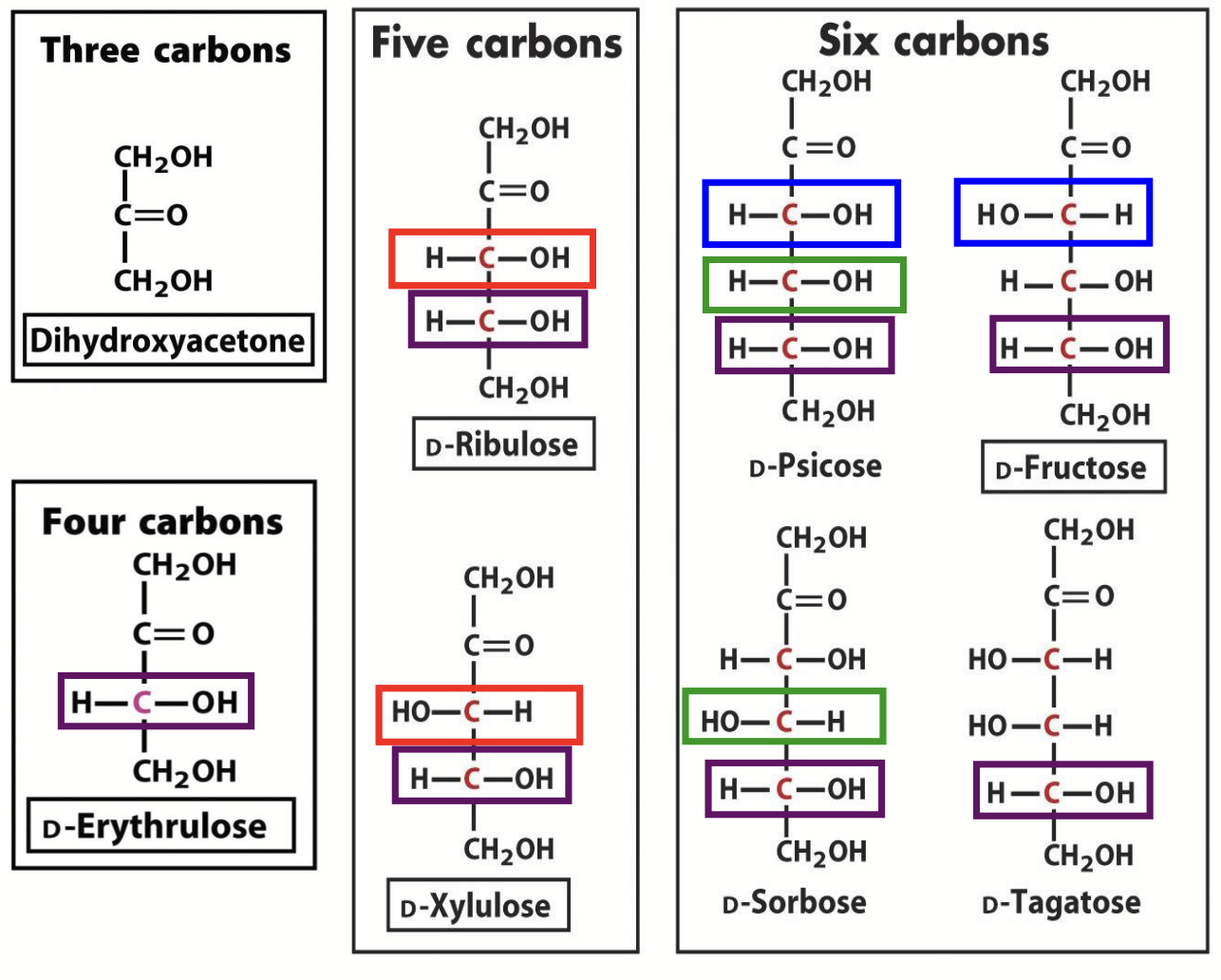
D-ketoses
Every time a C atom is added, the number of isomers doubles. This is why there are so many different simple sugars!
1 less chiral centre than aldose.
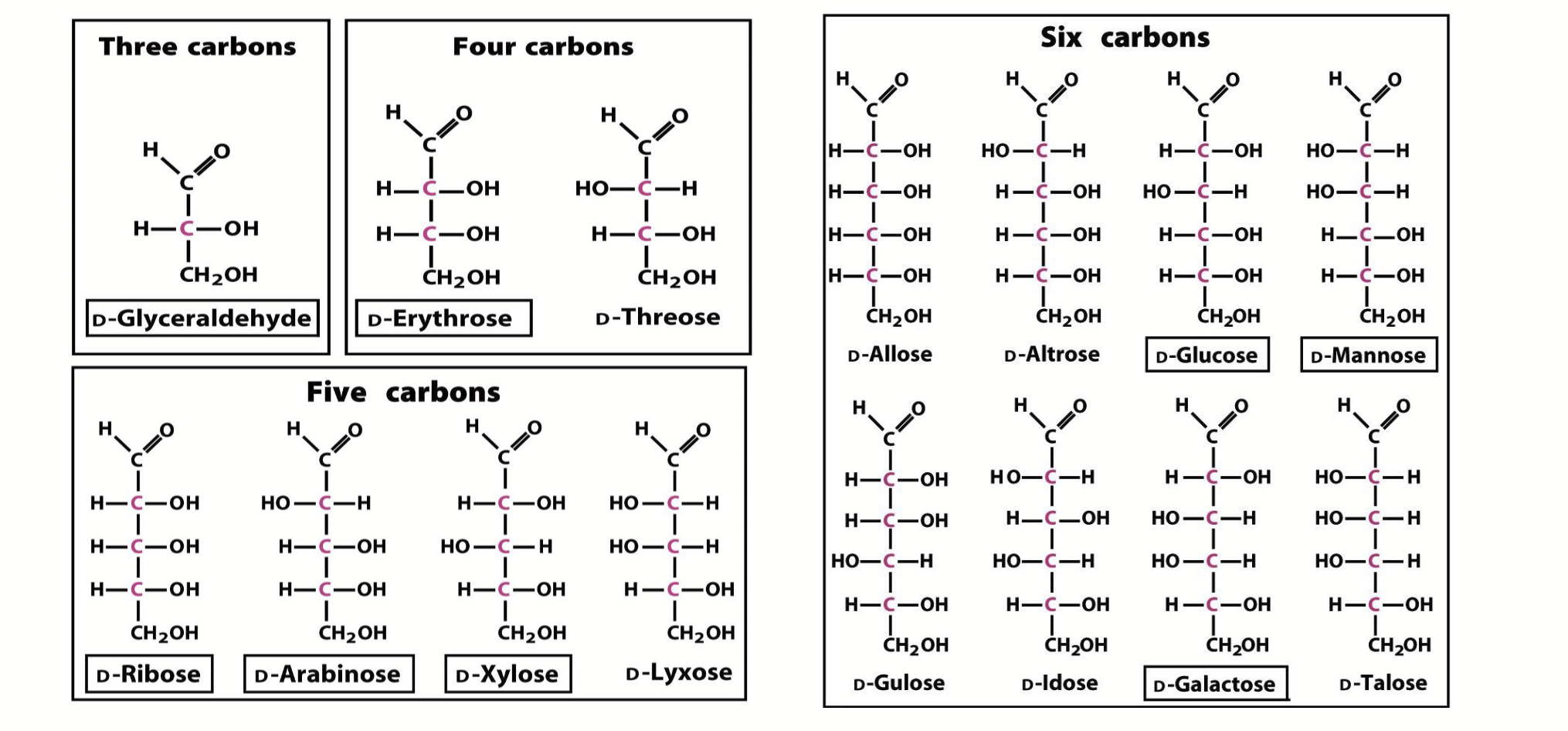
D-aldoses
1 more chiral centre than ketoses (isomeric forms determined by # chiral centres). There are always twice as many of these, compared to ketoses, for a given number of carbons.
hemiacetals
Aldehydes can react with alcohols.
Note: the original carbonyl carbon becomes chiral upon formation of these.
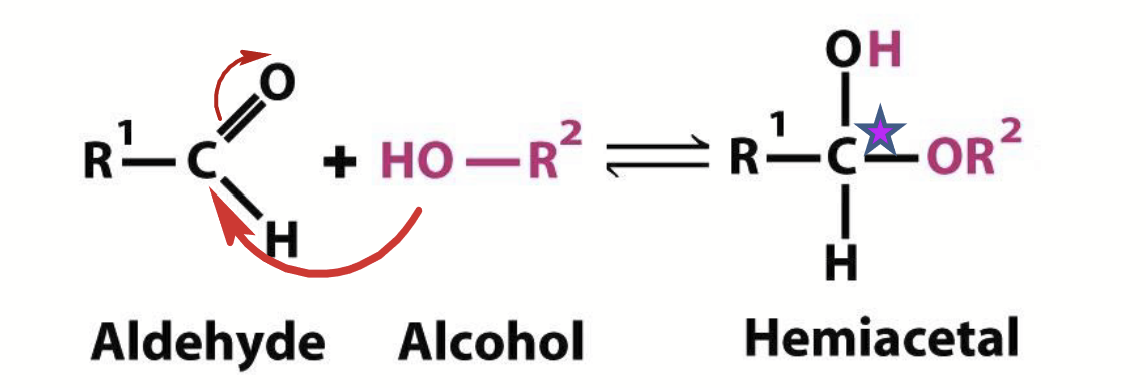
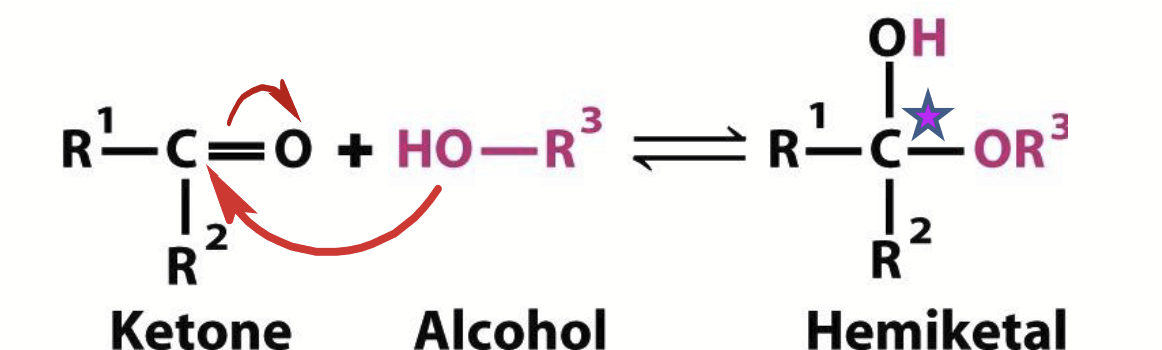
hemiketals
Ketones can react with alcohols.
Note: the original carbonyl carbon becomes chiral upon formation of these.
cyclization of sugars
Sugars have both alcohol and aldehyde or ketone functional groups.
Hemiacetal or hemiaketal formations are intramolecular: the alcohol and the aldehyde/ketone are present in the same molecule.
Hemiacteal and hemiketal formation turns sugars into ring structures.
cyclization of glucose
The OH group at C-5 reacts with the carbonyl carbon of the aldehyde group to form a stable six-membrane ring.
This renders C-1 asymmetric, giving rise to two stereoisomers alpha and beta.
These isometric forms that differ only in their configuration around the hemiacetal/hemiketal carbon are called anomers.
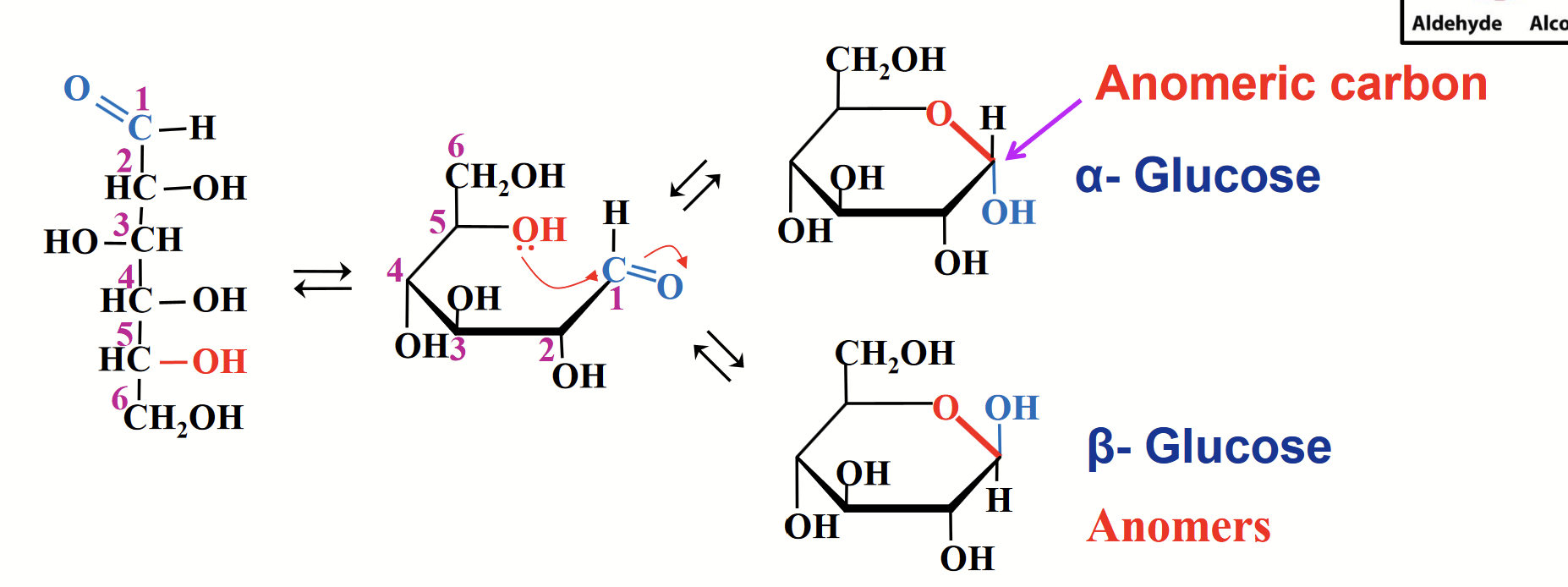
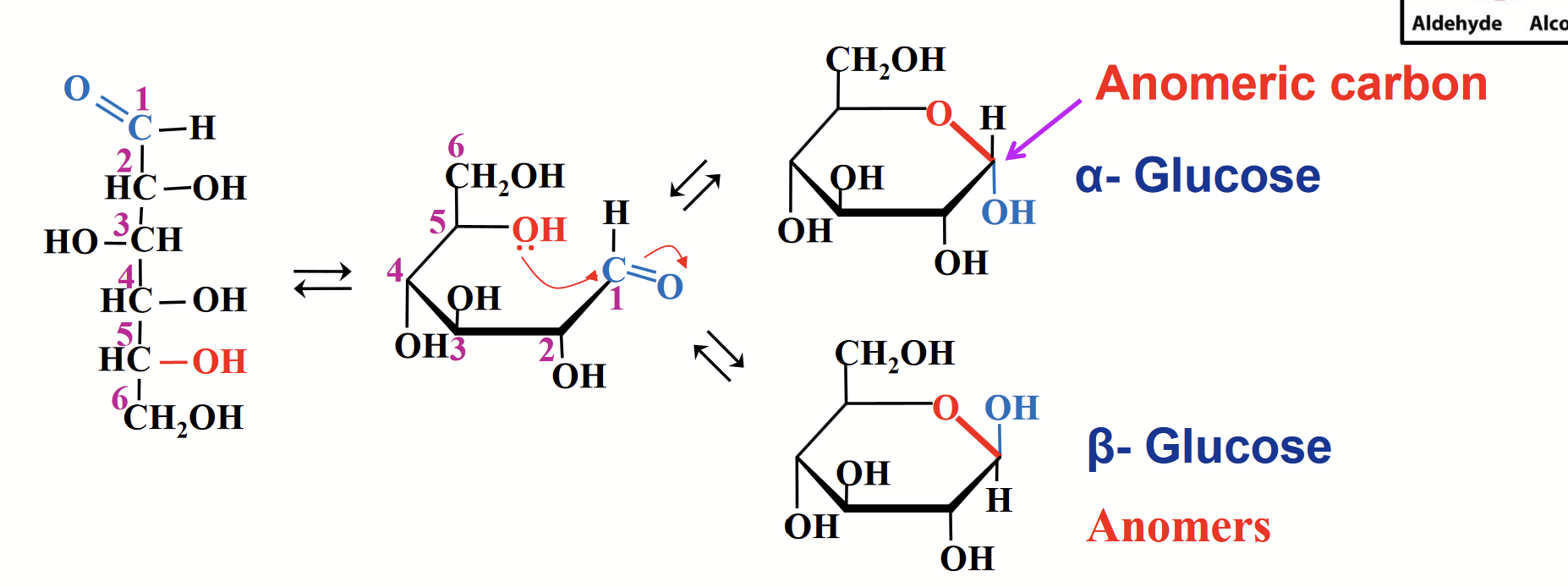
anomers
Isometric forms that differ only in their configuration around the hemiacetal or hemiketal carbon.
cyclization chemistry for a ketose
Follows the same theme as for an aldose. The electrophilic carbonyl carbon atom reacts with the nucleophilic O of an OH group.
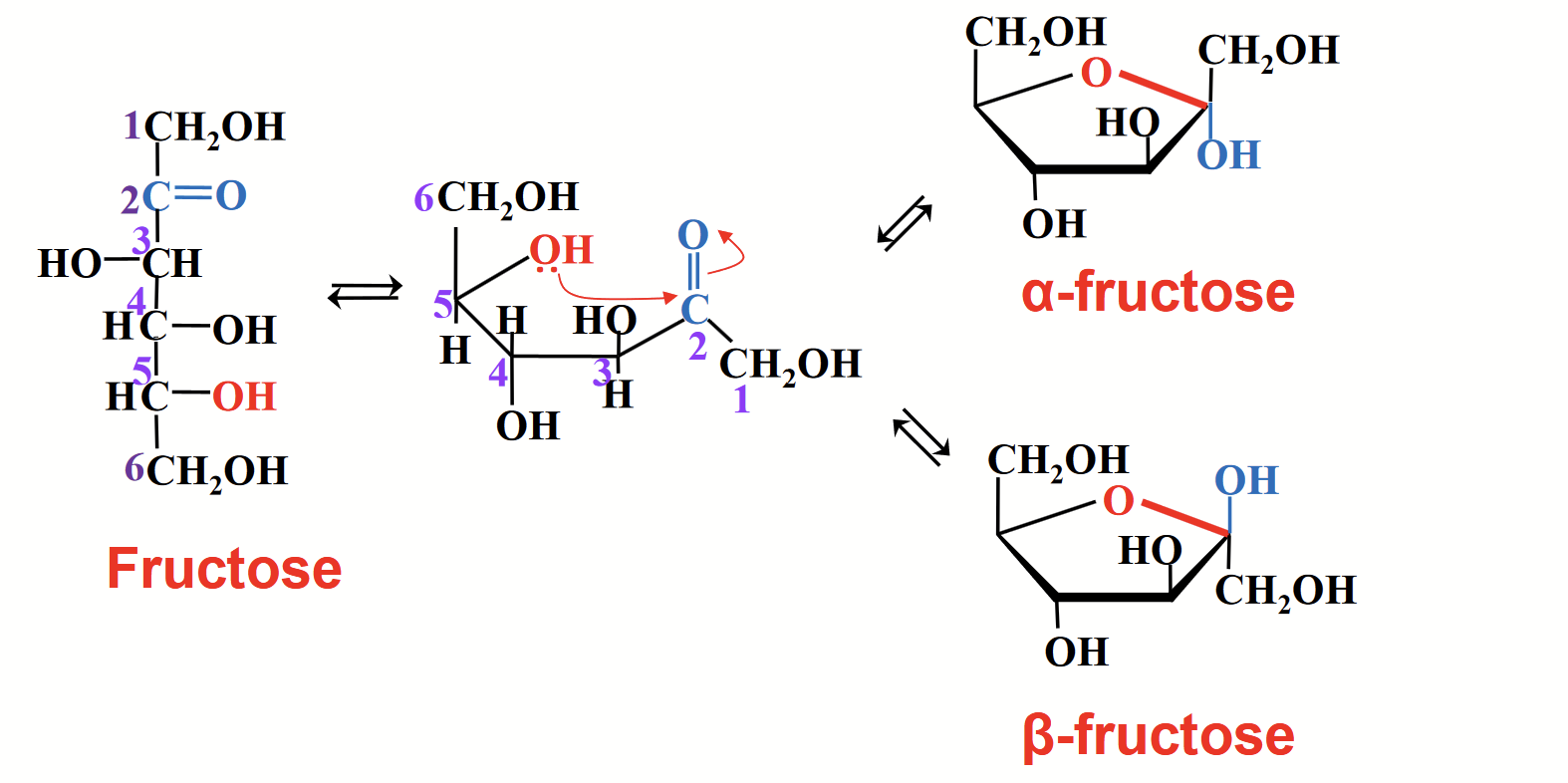
mutarotation
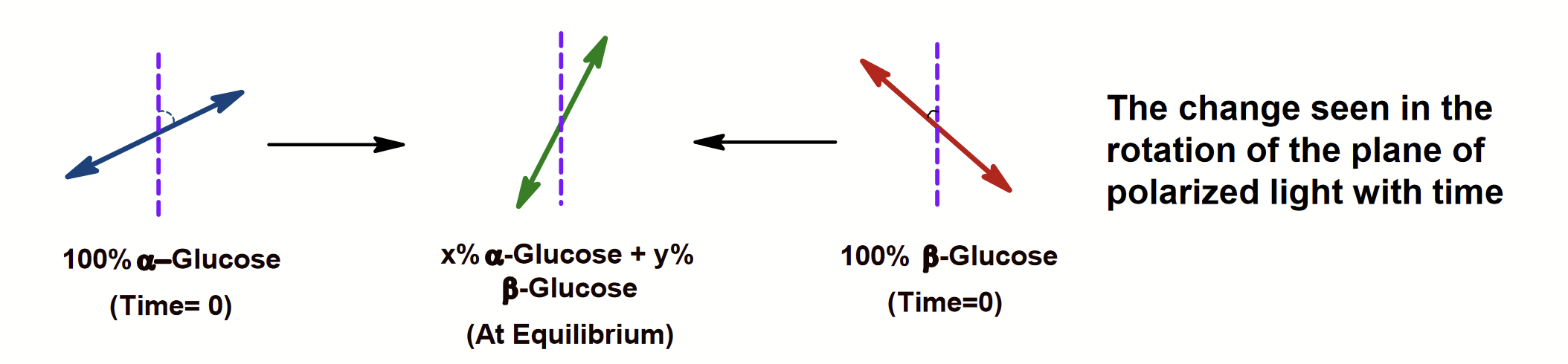
Solid glucose is found either in the alpha or the beta form of the hemiacetal. When dissolved in water, it slowly converts into an equilibrium mixture of the alpha, beta, and linear forms.
With time, a solution of alpha-D-glucose and a solution of beta-D-glucose form identical equilibrium mixtures with identical optical properties (approx. composition 1/3 alpha-D glucose; 2/3 beta-D glucose and trace amounts of the linear form).
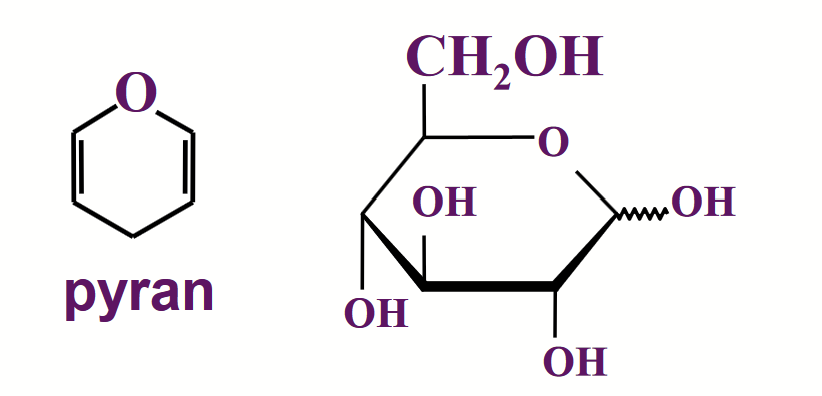
pyranose (6-membered ring)
The size of the ring formed by a particular sugar depends on the relative thermodynamic stabilities of the various possible ring structures, and this depends on the particular geometry of the molecule.
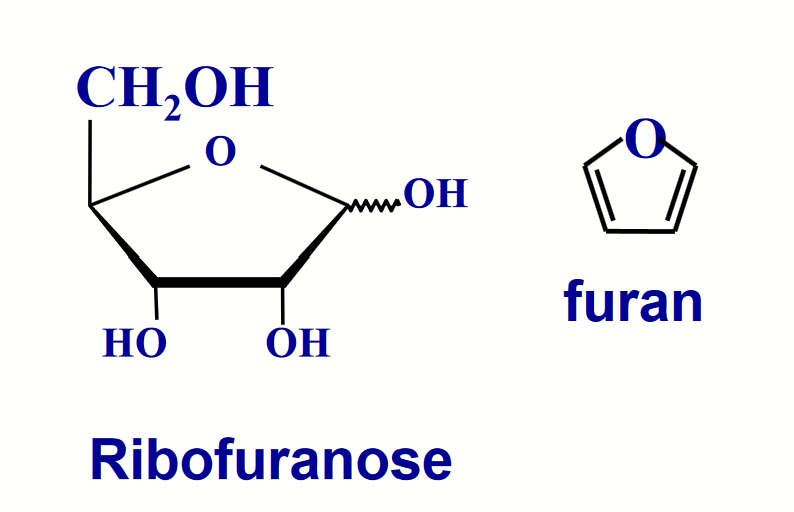
furanose (5-membered)
The size of the ring formed by a particular sugar depends on the relative thermodynamic stabilities of the various possible ring structures, and this depends on the particular geometry of the molecule.
reducing sugars
The carbonyl carbon of sugars undergo a redox reaction with cupric ion (Cu2+) under alkaline conditions. The carbonyl carbon is oxidized to a carboxylic acids and Cu2+ is reduced to cuprous ion (Cu+), forming a red precipitate.
This chemical property provides a simple basis for detecting the presence and concentration of a sugar like glucose (e.g. Fehling’s reaction).
Note: this redox reaction occurs only with the linear form (the form with the carbonyl carbon; the aldehyde or ketone form), which exists in equilibrium with the cyclic forms.
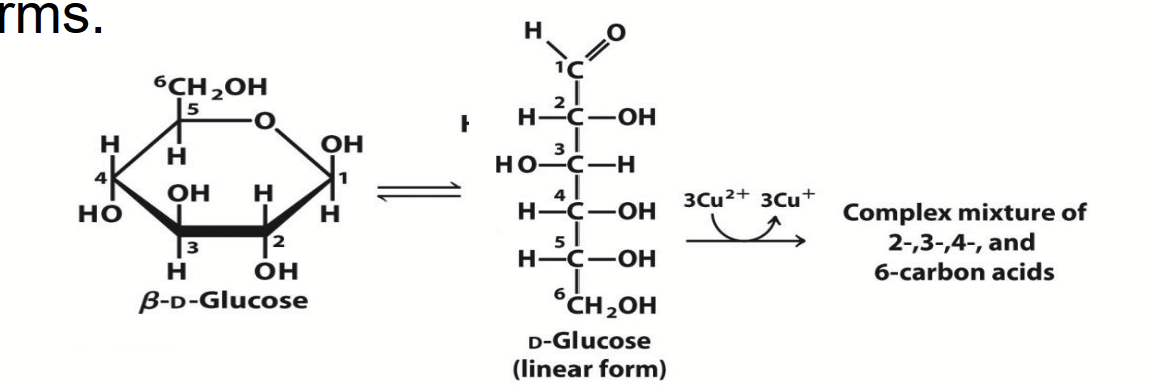
glycosides
The anomeric carbon atom of a sugar (the only carbon attached to two oxygen atoms) is electrophilic. This makes it reactive, and it is at this position that most reactions involving ring forms of sugars take place. The condensation of the anomeric carbon with the nucleophilic -OH of an alcohol or the -NH of an amine is the most important reaction of sugars.
The resulting molecule has a glycosidic bond (C-O) or a glycosilic (C-N) bond formed.
Examples: polysaccharides, nucleosides, the cardiac drug digoxin
glycosidic bond
A C-O bond in a glycoside.
If an anomeric carbon is associated in this bond, the sugar (in the ring form) can no longer open up to assume the lienar form.
non-reducing sugar
If the anomeric carbon is associated in a glycosidic bond, the sugar (in the ring form) can no longer open up to assume tha tlinear form.
Since only the open chain forms of sugars are able to undergo oxidation by cupric ions (reducing sugars), the sugar residue becomes a non-reducing sugar.
naming disaccharides
β1 →4
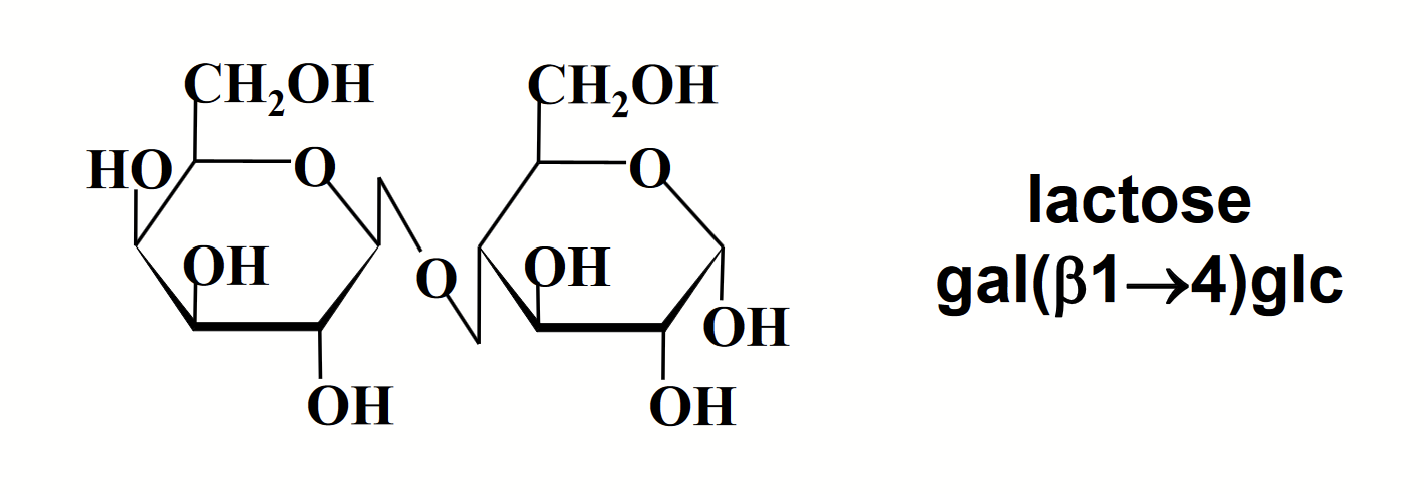
reducing and non-reducing disaccharides
Since the anomeric carbon of galactose (monosaccharide on the left) is involved in a glycosidic bond, it is unable to undergo mutarotation to form the linear form and becomes non-reducing.
The anomeric carbon of glucose (right monosaccharide) however is free and is able to undergo mutarotation existing in either α (α-lactose), β (β-lactose) or linear forms.
Lactose therefore is able to act as a reducing sugar.
In disaccharides and polysaccharides, the end of a chain with a free anomeric carbon (one not involved in a glycosidic bond) is called the reducing end.
reducing end
In disaccharides and polysaccharides, the end of a chain with a free anomeric carbon (one not involved in a glycosidic bond.
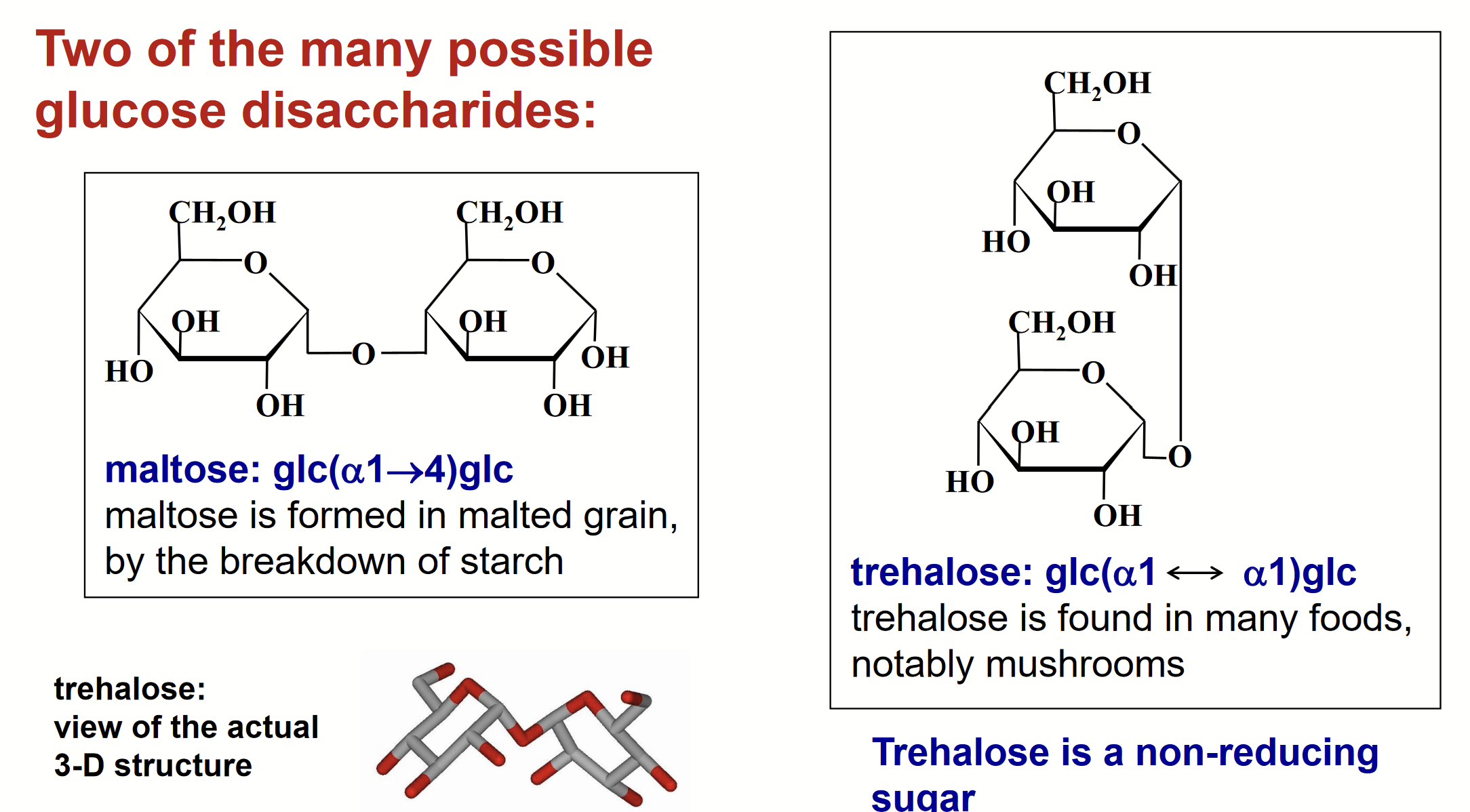
structural isomers of disaccharides
Formation of the glycosidic bond of a disaccharide must involve the anomeric C of one sugar, because that is the onyl electrophilic C atom in a sugar.
On the other hand, the -OH group (the nucleophile) coul be the OH on the anomeric C of a second sugar or any other -OH group in the sugar.
So, many disaccharides can be formed; they would be structural isomers.
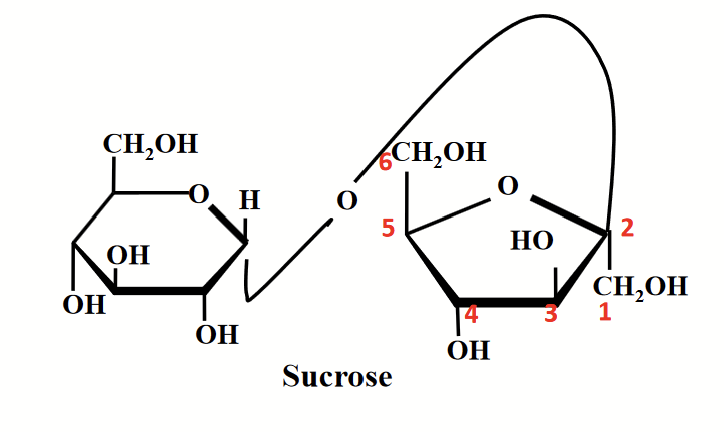
sucrose
Anomeric carbons of both glucose and fructose are involved in the glycosidic bond → non-reducing sugar.
Double-headed arrow: used to show the involvement of both anomeric carbon atoms in the glycosidic linkage.
homopolysaccharides
Polysaccharides made from a single type of monomer.
glucans: glucose homopolymers (e.g. starch, glycogen, cellulose, chitin)
mannans: mannose homopolymers
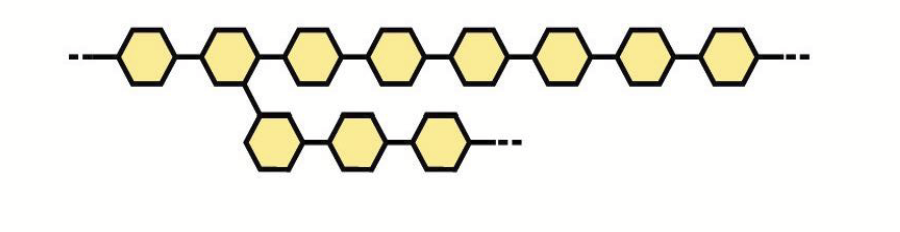
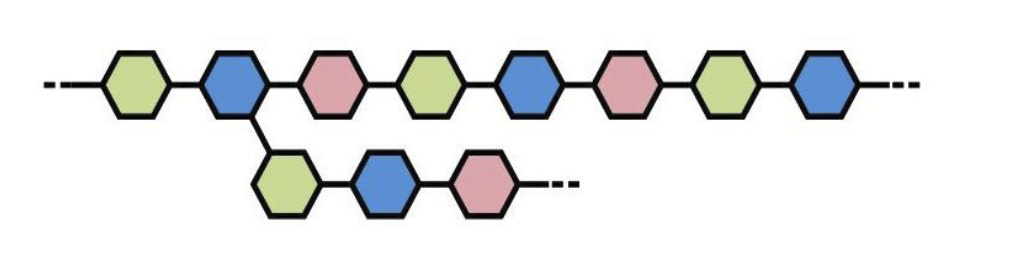
heteropolysaccharides
Polysaccharides made from two or more kinds of sugar subunits.
nucleic acids
The biochemical macromolecules that encode genetic information.
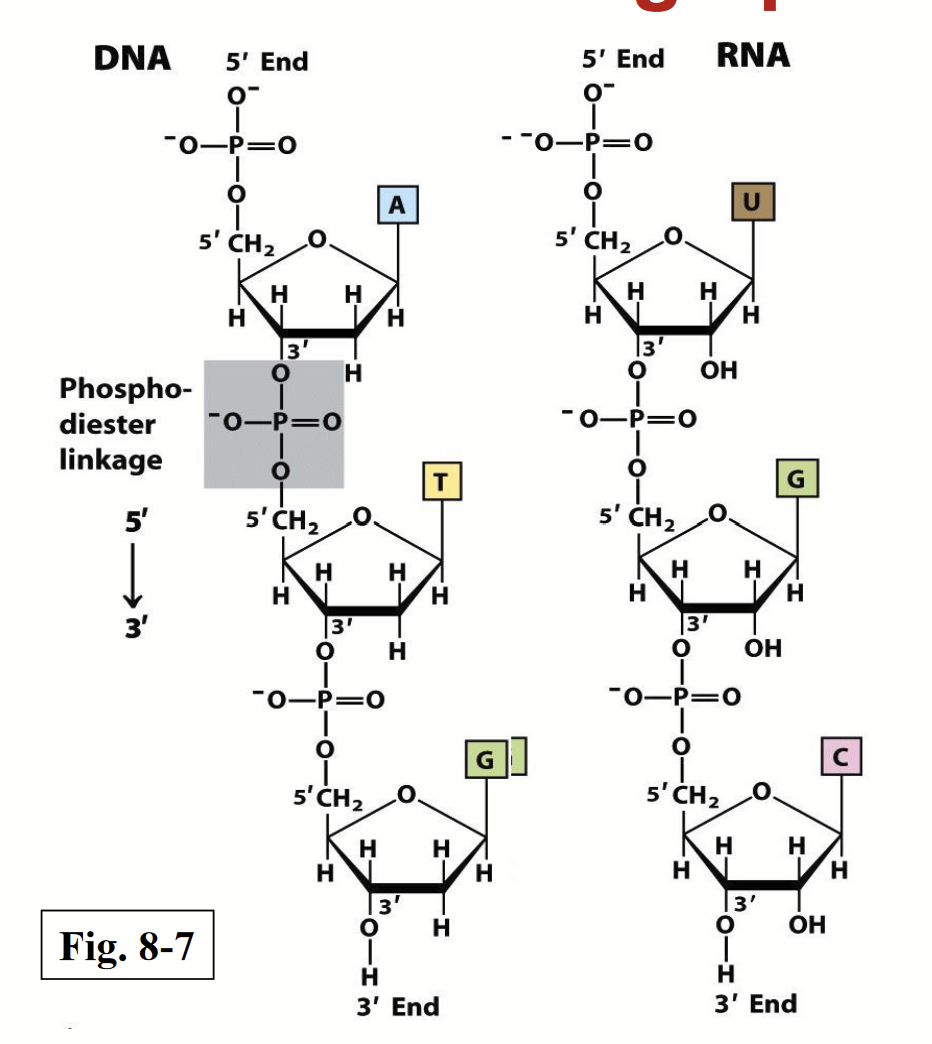
Linear polymers made by connecting nucleotides via phosphodiester bonds. Repeating unit is the nucleotide. The sugars, linked together by the phosphates, form the backbone - “sugar-phosphate backbone”.
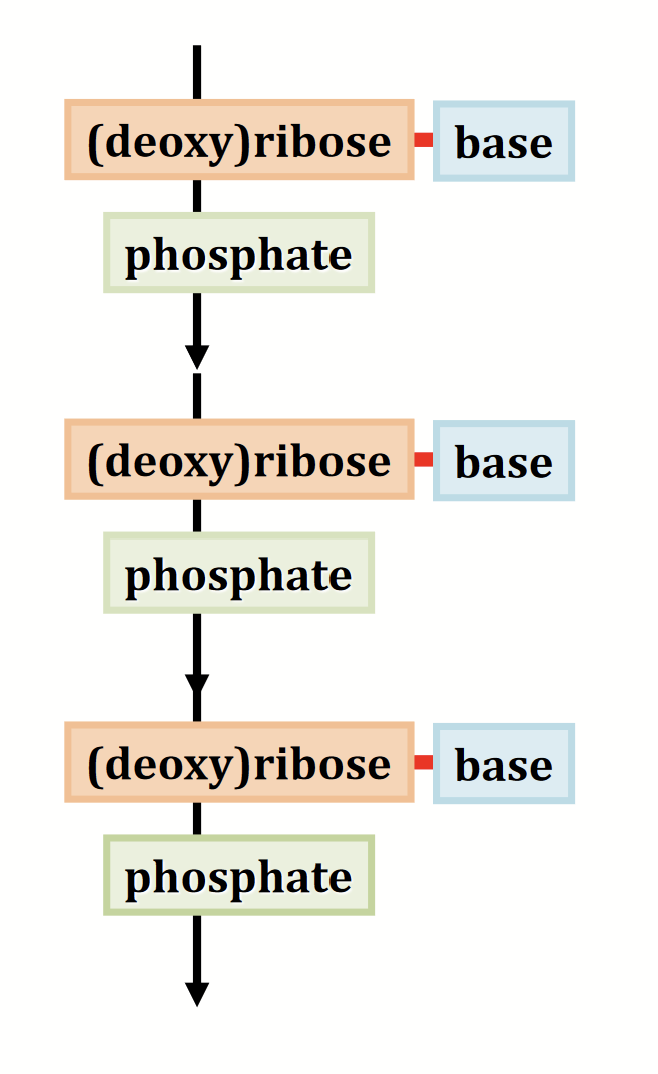
nucleotide
The repeating unit of nucleic acids. Consists of a sugar, a base, and a phosphate group.
Phosphorylated nucleosides.
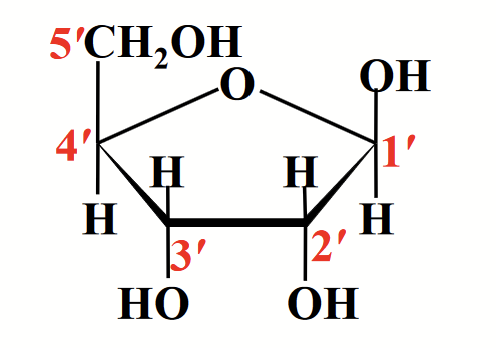
RNA
Nucleic acid that contains D-ribose.
Occurs in their beta-furanose form.
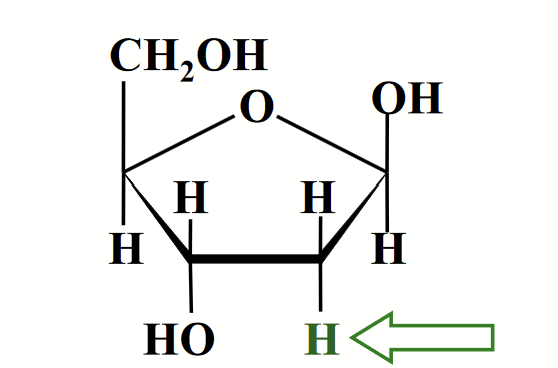
DNA
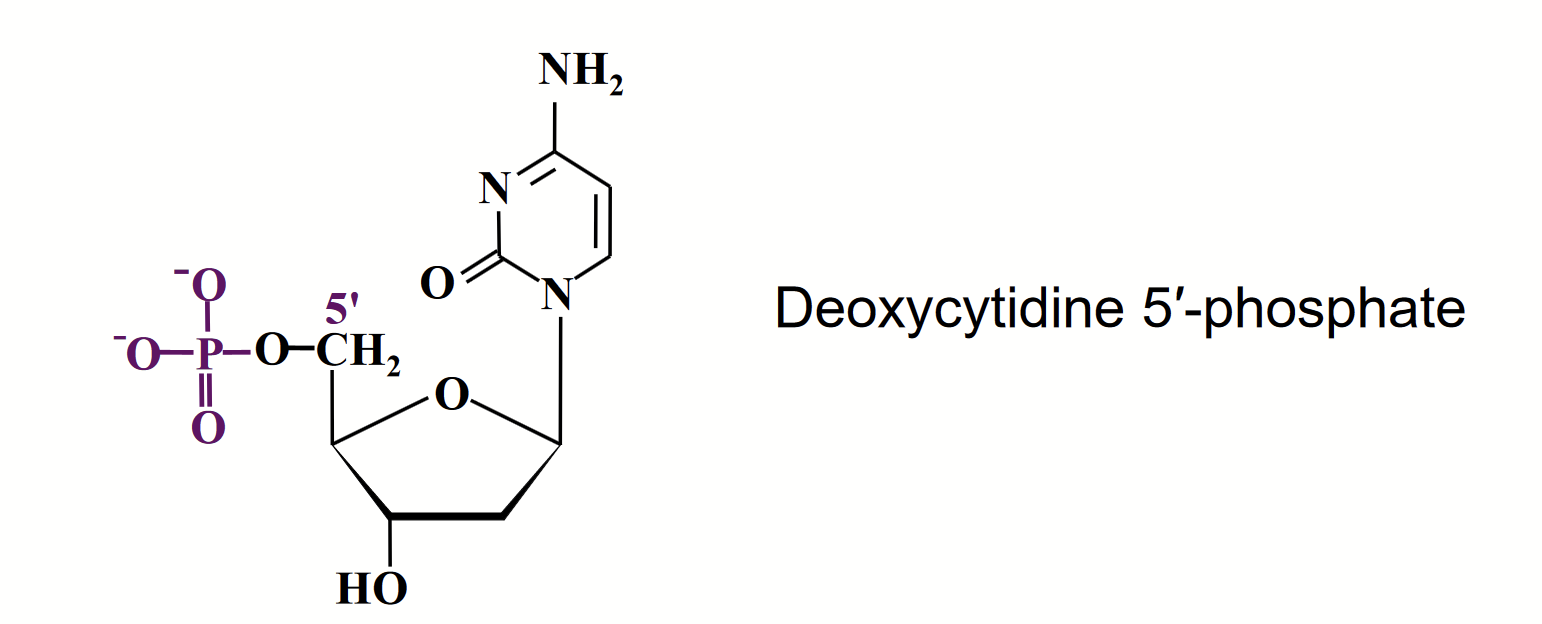
Nucleic acid that contains an unusual sugar, D-2-deoxyribose where the -OH of carbon 2 is replaced by -H.
Occurs in their beta-furanose form.
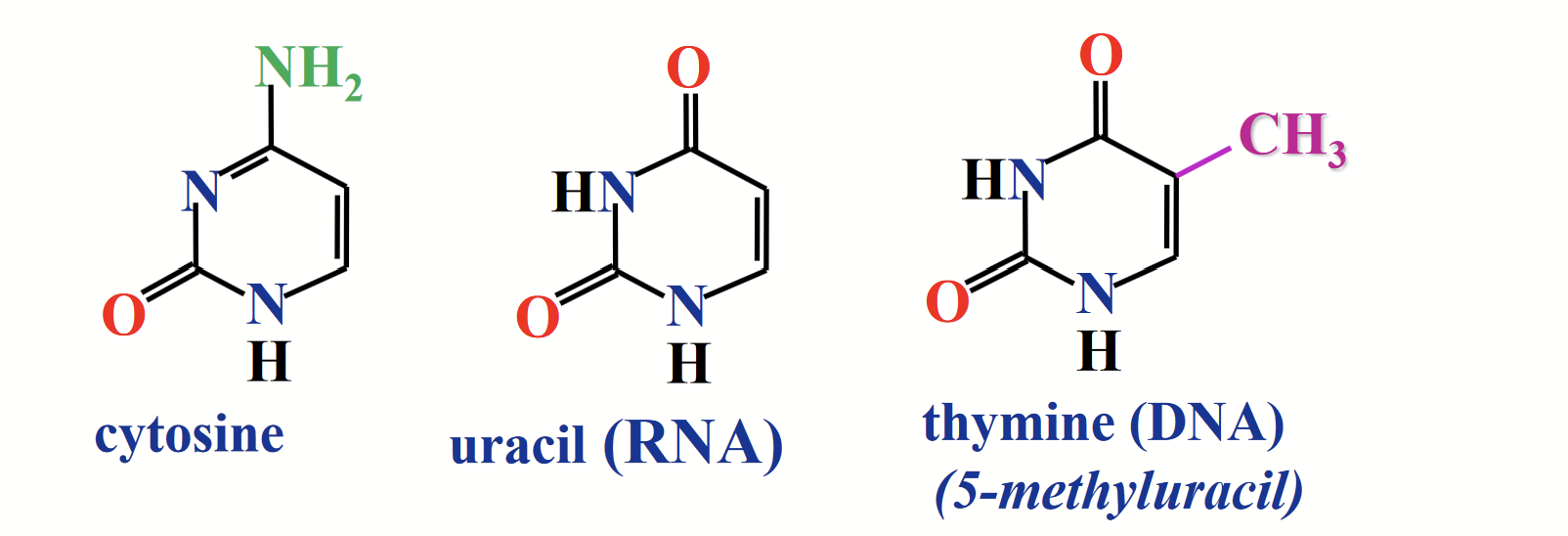
pyrimidines
Cytosine (C) and thymine (T) is in DNA and RNA. Uracil (U) is in RNA.
The NH at position 1 links to the anomeric carbon of ribose/deoxyribose.
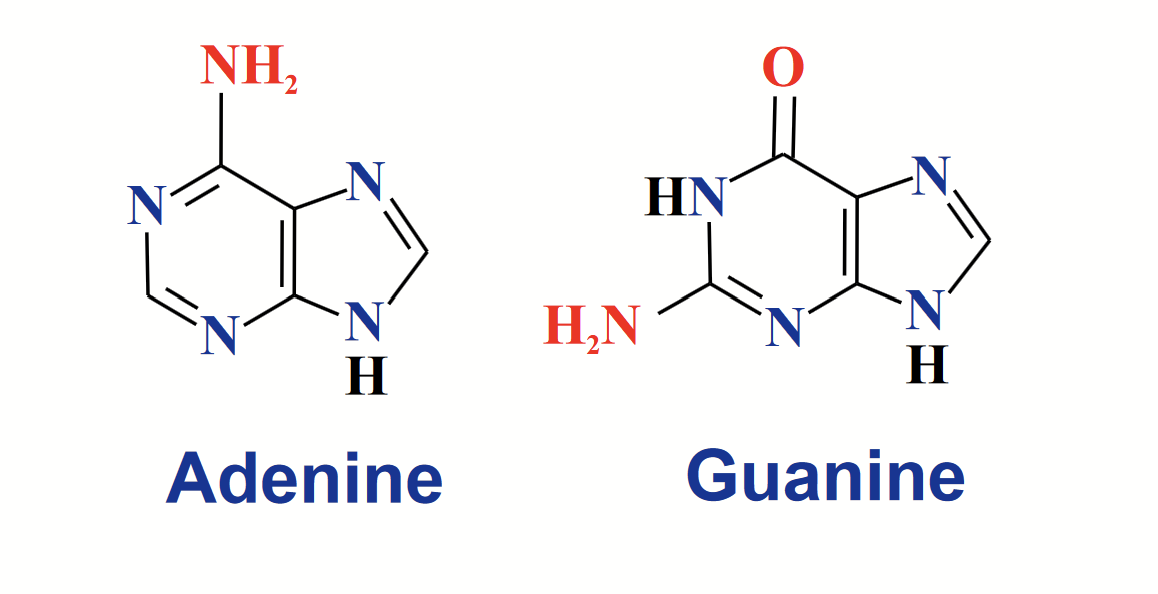
purines
A fused, bicyclic heterocycle.
Two bases in RNA and DNA: adenine (A) and guanine (G).
The NH at position 9 links to the anomeric carbon of ribose/deoxyribose.
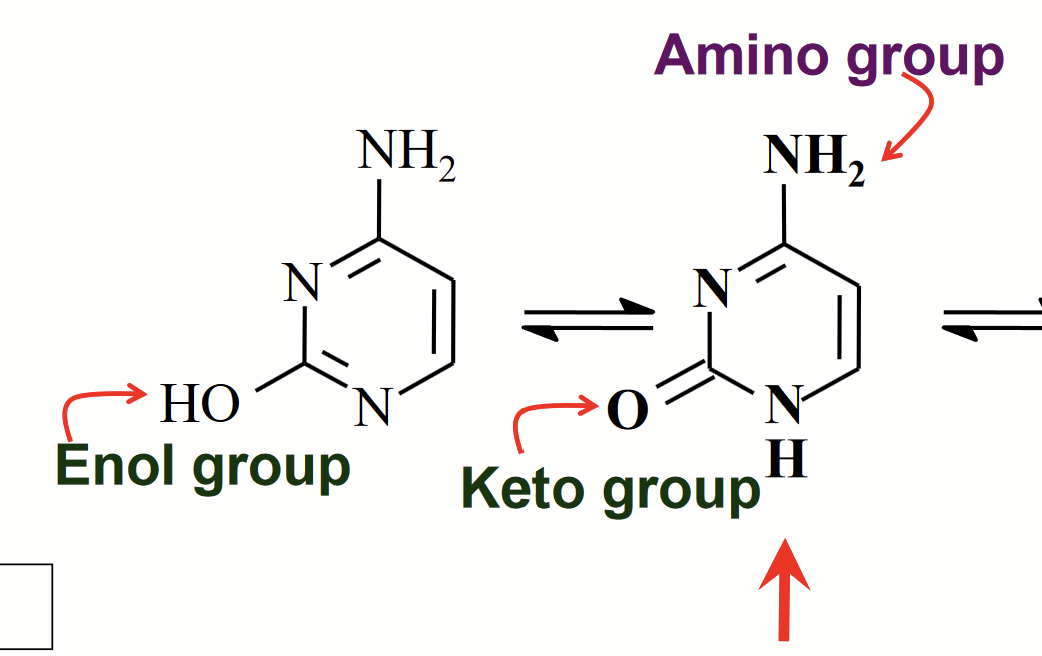
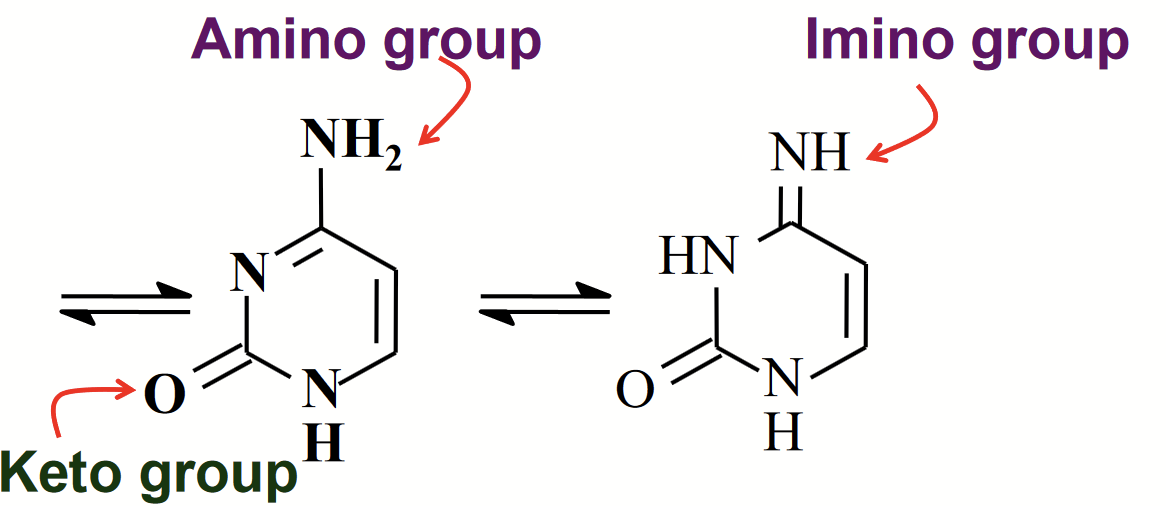
tautomeric forms of cytosine
Isomers that differ by the shift of an H atom and a double bond.
The OH group underges keto/enol tautomerism.
The NH2 group undergoes amino/imino tautomerism.
The form shown in the middle (amino + keto) predominates.
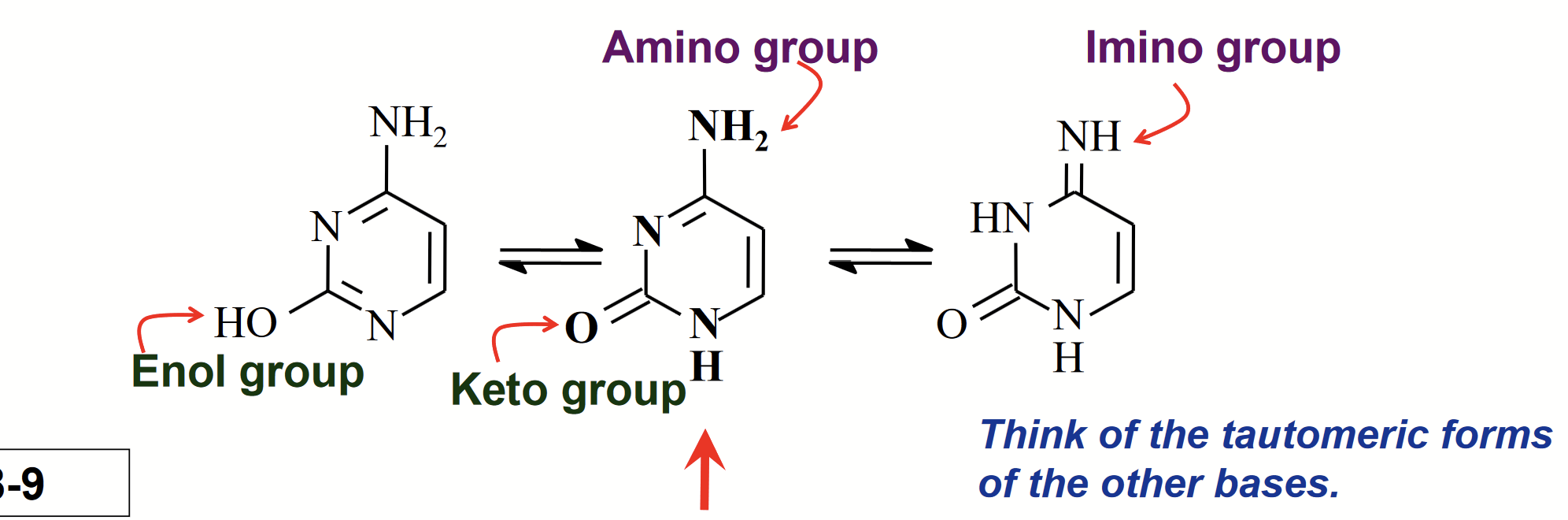
keto/enol tautomerism
The OH group undergoes tautomerism for cytosine.
amino/imino tautomerism
The NH2 group undergoes tautomerism.
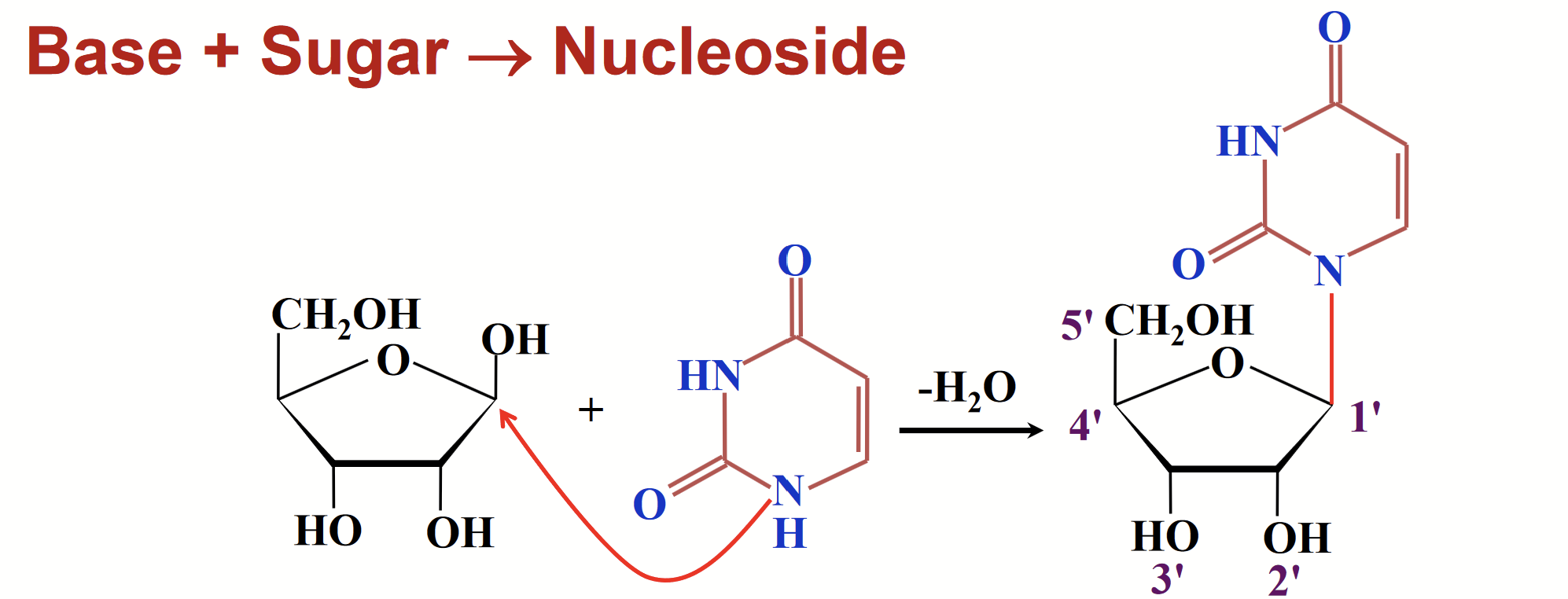
nucleoside
A special type of glycoside found in nucleic acids; a base is joined to the sugar through a glycosidic bond.
The glycosidic bond is sometimes called a glycosylic” bond to designate the C-N linkage.
phosphodiester linkage
A phosphate group bridges between the 5’ OH of one nucleotide unit and the 3’ OH of another. The linkage pattern is the same for both DNA and RNA.
Each linear nucleic acid strand has a specific 5’ end (lacks a nucleotide at the 5’ position) and a 3’ end (lacking a nucleotide at the 3’ position).
The phosphate groups are completely ionized and negatively charged at pH 7.
Nucleotide sequences are written from 5’ → 3’.