Lecture 4 - Tertiary Structure/Protein Purification
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
53 Terms
Tertiary Structure
Folding of secondary elements for a complete 3-D structure of one polypeptide, allowing distant amino acids to be closer
Interactions stabilizing tertiary structure
H-bonding, covalent bonds, salt bridges, LDFs, hydrophobic effect between backbone AND side-chains
What structure is most favorable?
Primary: highest entropy
What structure is least favorable?
Tertiary: lowest entropy due to folding
What is the strongest force in protein folding?
The hydrophobic effect (though it’s still not enough to make it favorable)
Hydrogen Bonding in Tertiary Structure
Side-chain to side-chain
Backbone to backbone
Side-chain to backbone
Salt bridges (ion-pairing)
Interaction between + and - charged amino acids
Positively charged amino acids
Lysine, histidine, arginine
Negatively charged amino acids
Aspartate, glutamate
van der Waals (LDF) Interactions
Weak electrostatic between fixed/induced dipoles; strong collectively
What creates van der Waals forces?
Attraction between electron-rich and poor regions of two molecules (electrons attract to positive nucleus)
Hydrophobic Effect
Increases entropy of water due to non-polar R-groups distributing to the interior of molecule
What increases entropy in protein folding?
The hydrophobic effect
What decreases enthalpy in protein folding?
Many van der Waals forces
Disulfide Bonds
Covalent bonds between cysteine residues (reversible)
Serum Albumin
Protein containing many disulfide bonds
Where is information needed to fold protein contained?
Primary structure (linear amino acid sequence)
How do we know primary sequence holds folding information?
If we denature protein with BME and Urea, the protein can refold IF done in the correct process of removing urea, then oxidizing
Role of BME
Denature protein by reduction (adding hydrogen)
Role of urea
Denatures protein by breaking H-bonds (unfolding)
Steps to mis-folded protein
Oxidize (reform disulfides randomly)
Remove urea (refolding, H-bonds)
Steps to correctly folded protein
Remove urea (H-bonds, refolding)
Oxidize (reform disulfides)
SPECIFIC steps, so that disulfides form in correct places
Temperature increase causes..
Unfolding of proteins (denaturing)
Temperature decreases causes..
Proteins to refold
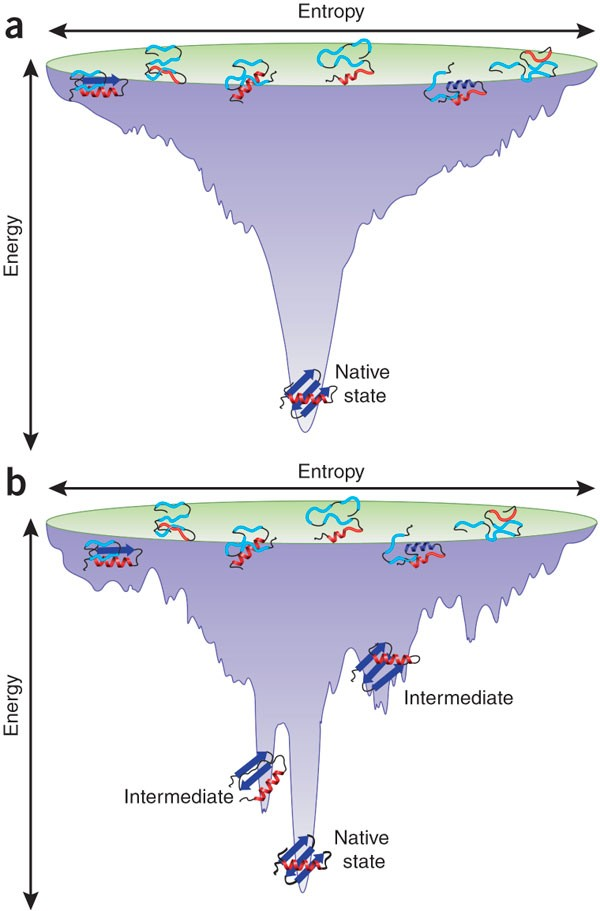
Energy Landscape of Proteins
Like a funnel; denatured are high entropy, folded are low entropy with many different conformations
Fibrous Proteins
Rod-shaped proteins that provide structure, organized as high-order arrays of secondary structures (helices/sheets)
What shape are fibrous proteins?
Rods
What is the function of fibrous proteins?
Structure
Motifs
Repeating sequence units (often in fibrous proteins)
Examples of fibrous proteins
a-Keratins, fibroin, collagen
a-Keratins are found in…
Hair, wool, nails
Fibroin is found in…
Silk
Collagen is found in…
Connective tissues, bones, tendons, vessels, etc.
Diversity of Fibrous Proteins
Is limited because of motifs; often primarily 3 amino acids
a-Keratin Motif
7-residue repeat (abcdefg)n where a and d are hydrophobic
Fibroin Motif
(Ser-Gly-Ala-Gly)n
Tropocollagen Motif
(Gly-X-Y)n
Structure of a-keratins
All a-helical with pseudo-repeat motif
(abcdefg)
Hydrophobic a/d positions form a sticky strip, creating a coil of coils
Higher order structure = VERY stable
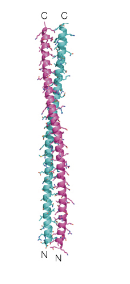
Covalent bonding in a-keratins
Disulfide bonds can be broken and rearranged (reduced then oxidized); used in perms
What bond stabilizes a-keratins?
Disulfide bonds
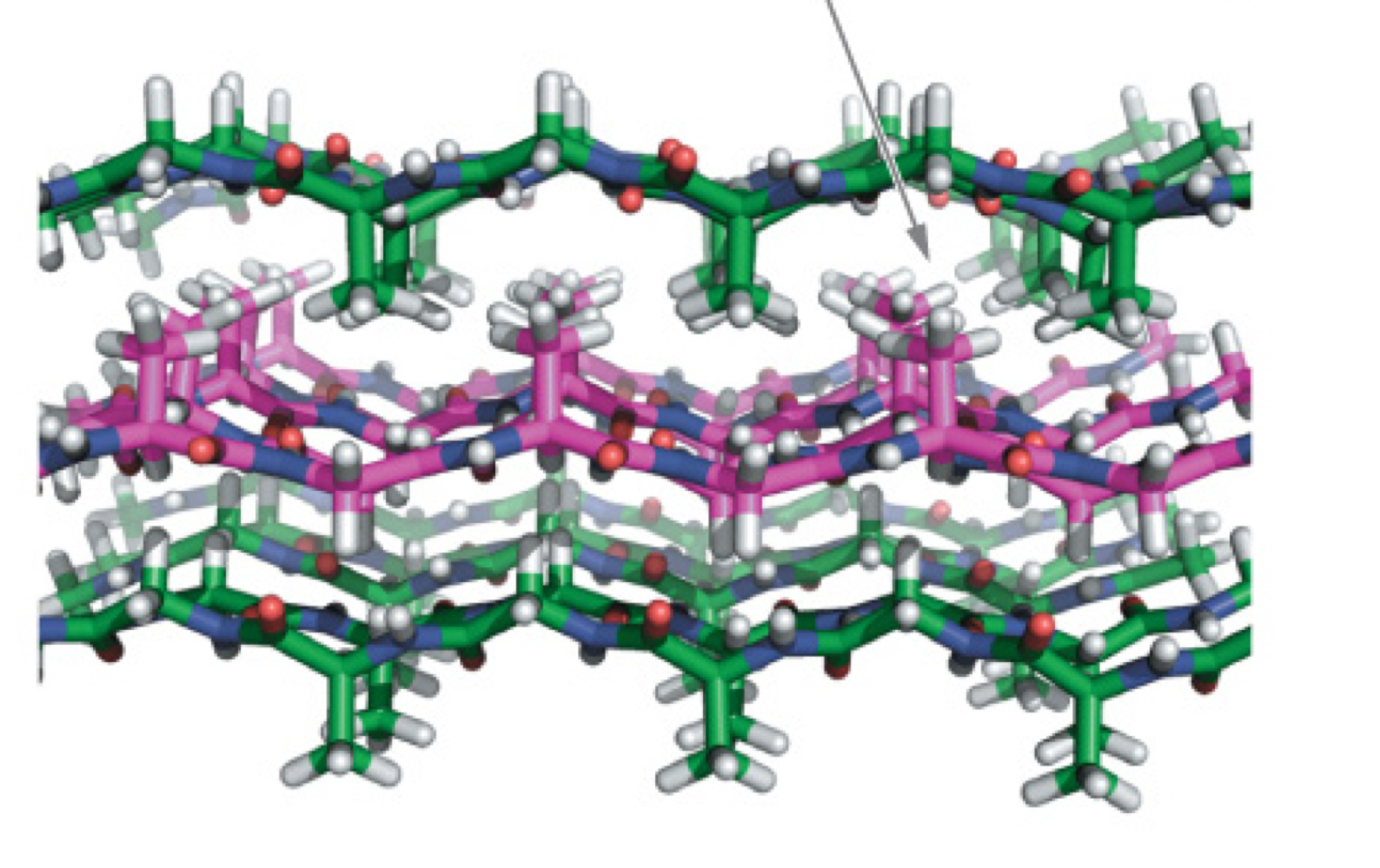
Fibroin Structure
Antiparallel beta-sheet
Repeating motif: (Ser-Gly-Ala-Gly)n
All small amino acids
Side chains between adjacent sheets alternate between big and small
Interdigitate to allow for stable packing
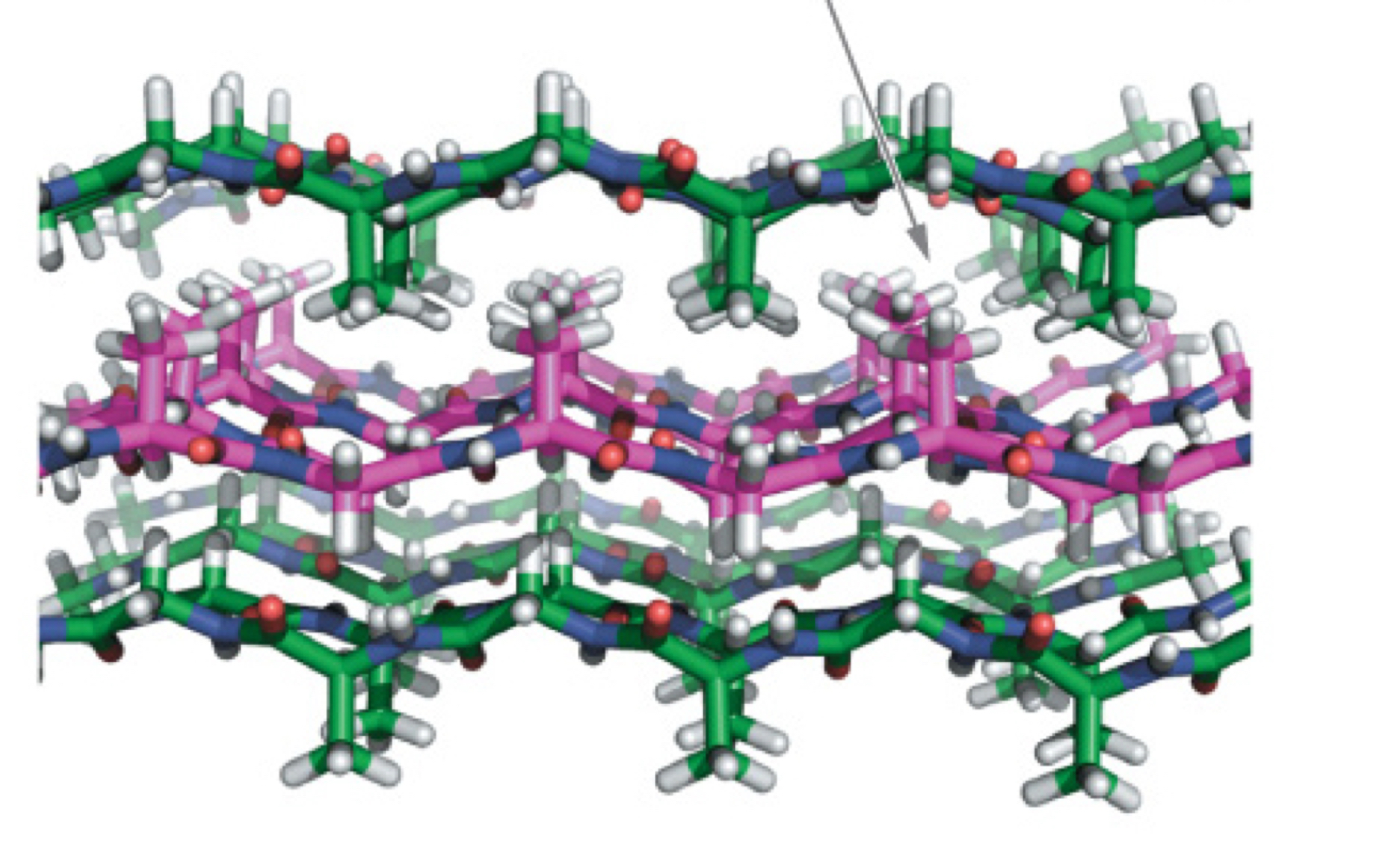
Fibroin Packing
Antiparallel b-sheets are already extended and VERY strong; flexible because inter-sheet van der Waal forces are weak (slide between)
Tropocollagen Structure
Left-handed helix (NOT alpha-helix) with 3 residues per turn
Gly-X-Y motif
X = proline, Y = hydroxyproline
H-bonding INTER-chain, NOT intra-chain
Peptide bonds perpendicular to plane
Tropocollagen Superhelix
3 interwound helices twisted right-handed
H-bonds stabilize between amide hydrogens and carbonyl oxygens
Glycine is in the central position
Post-translational Modifications
Cross-linking
Hydroxylation of proline
Hydroxylation of lysine
ALL used to stabilize collagen
Cross-linking
Post-trans. modification requiring Cu2+ for lysyl oxidase activity to stabilize collagent
Cross-linking increases with…
Age - loss of elasticity and brittle bones
What two substances are needed for cross-linking?
Cu2+ and lysyl oxidase
Copper deficiency leads to..
Weakened collagen (affecting bone, tendons, muscles)
Hydroxylations
Post-trans. modifications that stabilize collagen by hydroxylating proline or lysine
Enzymes for hydroxylation
Proline hydroxylase, lysine hydroxylase
What other molecule is needed for hydroxylation?
Ascorbate (Vitamin C)
Vitamin C Deficiency (Scurvy)
Leads to weak collagen due to its role in hydroxylation