Chemistry CC8
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
The pH scale
0 - 6 = Acids
7 = Neutral
8 - 14 = Alkalis
0 = Most acidic
14 = Most alkaline
Acids
Form H+ ions in water
Higher H+ concentration - lower pH
Alkalis
Form OH- ions in water
Higher OH- concentration - higher pH
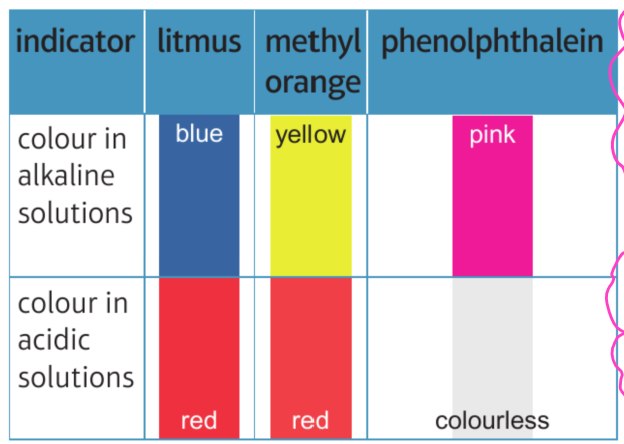
Indicators
Indicators go different colours due to the pH
Acid reactions (hydrogen)
To test for hydrogen
lighted splint
H2 gas in test tube
Put the lit splint into the test tube
If hydrogen is present there will be a squeaky pop
Acid reactions (Carbon dioxide)
To test for carbon dioxide
Limewater
Gas (CO2)
Bubble the gas through the limewater
If the limewater goes cloudy then Carbon dioxide is present
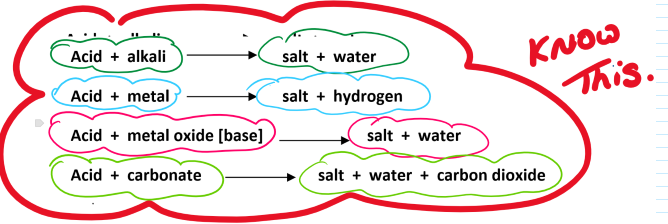
Reactions of acids table
Strong and weak acids
Strong acid
An acid that completely ionises in water to produce hydrogen ions. Some examples are - hydrochloric acid, sulfuric acid, and nitric acid.
Weak acid
An acid that partially ionises in water to produce hydrogen ions. Some examples are - Ethanoic acid, Citric acid, and Carbonic acid.
Acid strength and concentration
Strength
The proportion of acid molecules that ionise in water.
Concentration
The number of acid molecules in a certain volume of water.