Chem- Ch 8
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms

“yeilds” indicates result of reactions
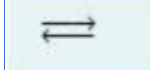
used in place of a single arrow to indicate a reversible reaction

reactant/product in solid state: alsi used to indicate a precipitate

alt to (s) but used ONLY to indicate precipitate

reactant or product in liquid state
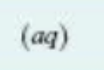
reactant or product in an aqueous solution (dissolved in water)

reactant or product in gaseous state

alternative to (g) but only in gaseous PRODUCT
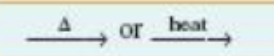
reactants heated

pressure at which reaction is carried out

pressure at which reaction is carried out that exceeds normal atomspheric pressure

temp at which reaction is carried out
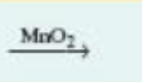
formula of catalyst, in this case maganese dioxide, used to alter the rate of the reaction
chemical reaction-
the process by which one or more substances are changed into one or more different substances.
Signs of chemical reaction:
Heat and light emitted
Production of a gas
Formation of a precipitate
Color change
Diatomic molecule
elements that contain 2 atoms
EXAMPLES of diatomic molecules
H2, N2, O2, F2, Cl2, Br2, I2
Law of conservation of mass-
the total mass of the reactants must equal the total mass of products for any given chemical reaction.
Coefficients are used to…
equalize the number of atoms
Coefficient-
small whole number in front of a chemical formula
Coefficients indicate relative,
not absolute, amounts of reactants and products
Relative mass of products/reactants is determined
from reactions coefficients
word equations dont…
Does not give amounts of reactants or products formed
formula equation-
represents the reactants and products of a chemical equation by their symbols or formulas.
A balanced formula equation gives both…
qualitative and quantitative info.
Synthesis-
when two or more substances combine to form one new product.
Decomposition-
A single compound undergoes a reaction that produces 2 or more simpler substances.(opposite of a synthesis reaction)
**CAN ONLY TAKE PLACE WHEN ENERGY IN THE FORM OF ELECTRICITY OR HEAT IS ADDED**
Single displacement-
One element replaces a similar element in a compound.
Double displacement-
The ions of two compounds exchange places in an aqueous solution to form two new compounds.
Combustion-
A substance combine with oxygen releasing a large amount of energy in the form of light and heat (starts with O and produces CO2 and H20)