P-8 Gels and Liposomes
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
What are gels?
Viscoelastic, solid-like materials comprised of an elastic cross-linked network and a solvent.
What is the solid-like appearance of a gel the result of?
Entrapment and adhesion of the liquid in the large SA of a solid 3D matrix.
What caused the formation of solid matrix in a gel?
Cross-linking of polymeric strands of macromolecules by physical/chemical forces.
Why do concentrated polymer solutions often show high viscosity?
Bc of 3D interaction of polymer chains w/solvent.
What are gels characterised by?
Critical polymer conc (below which gel is not formed).

List some characteristic features of gels.
- Large increase in viscosity above gel point.
- Appearance of rubber-like elasticity.
- Gel retains shape under low stress but deforms at higher stress.
- Can respond to environment(pH/light).
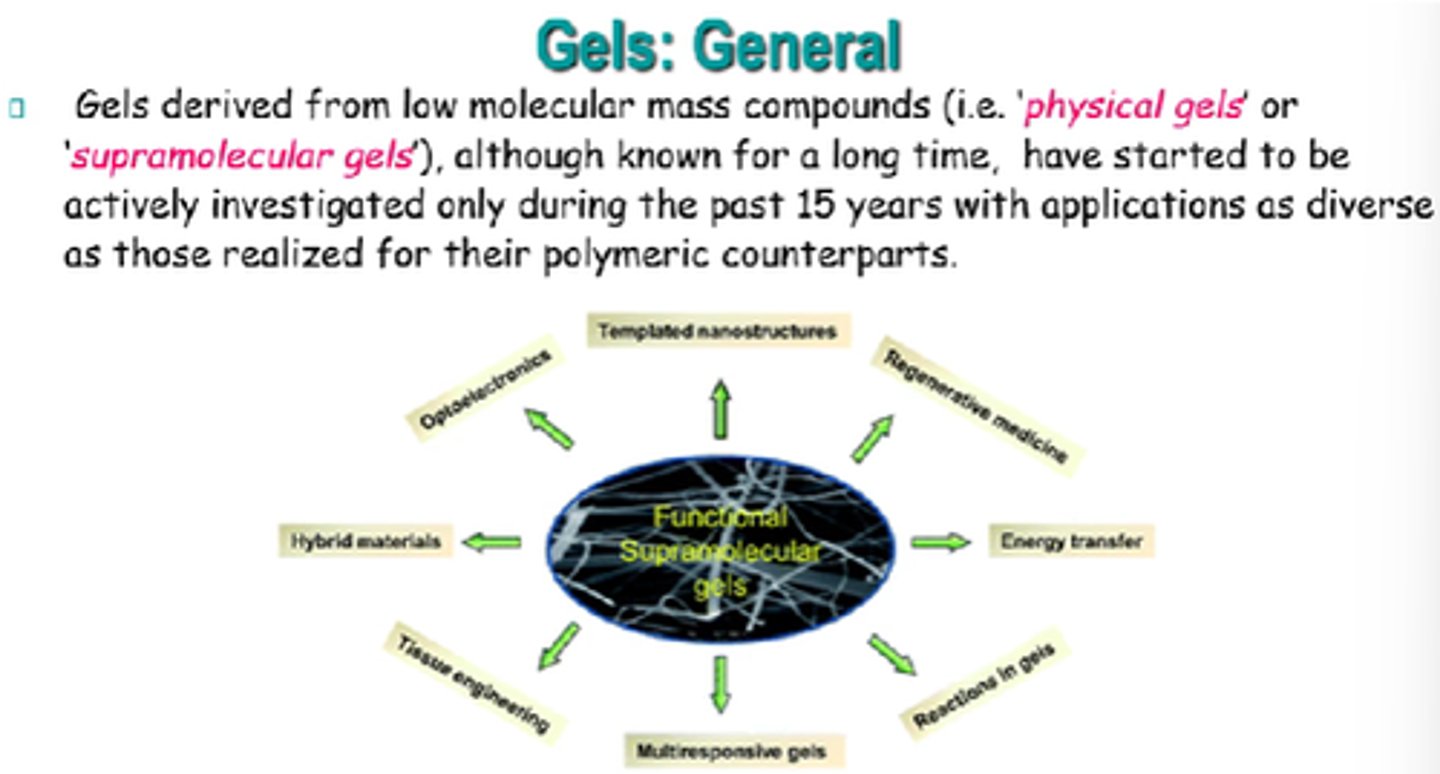
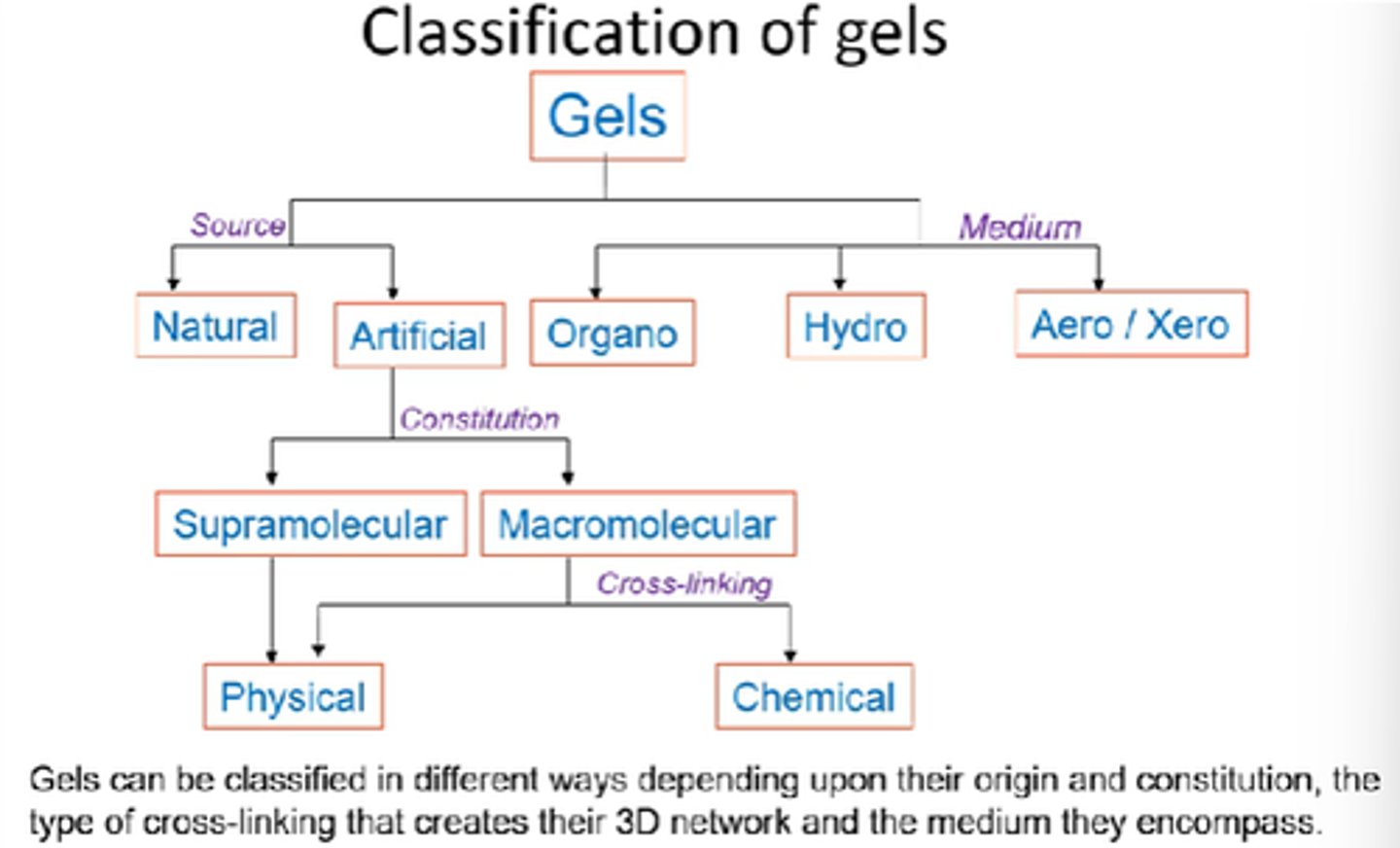
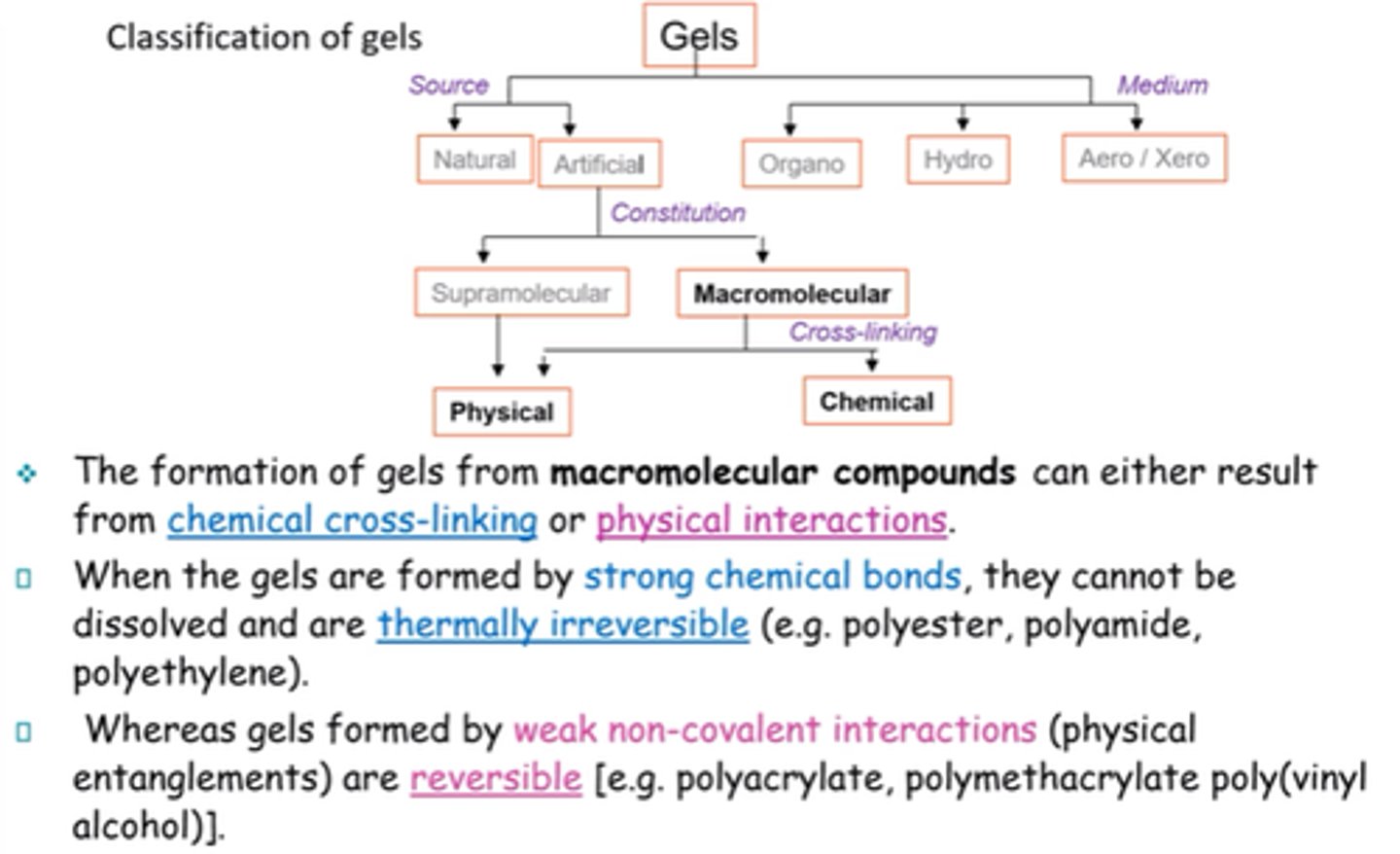
What are the 2x main classifications of gels?
Source/origin and medium
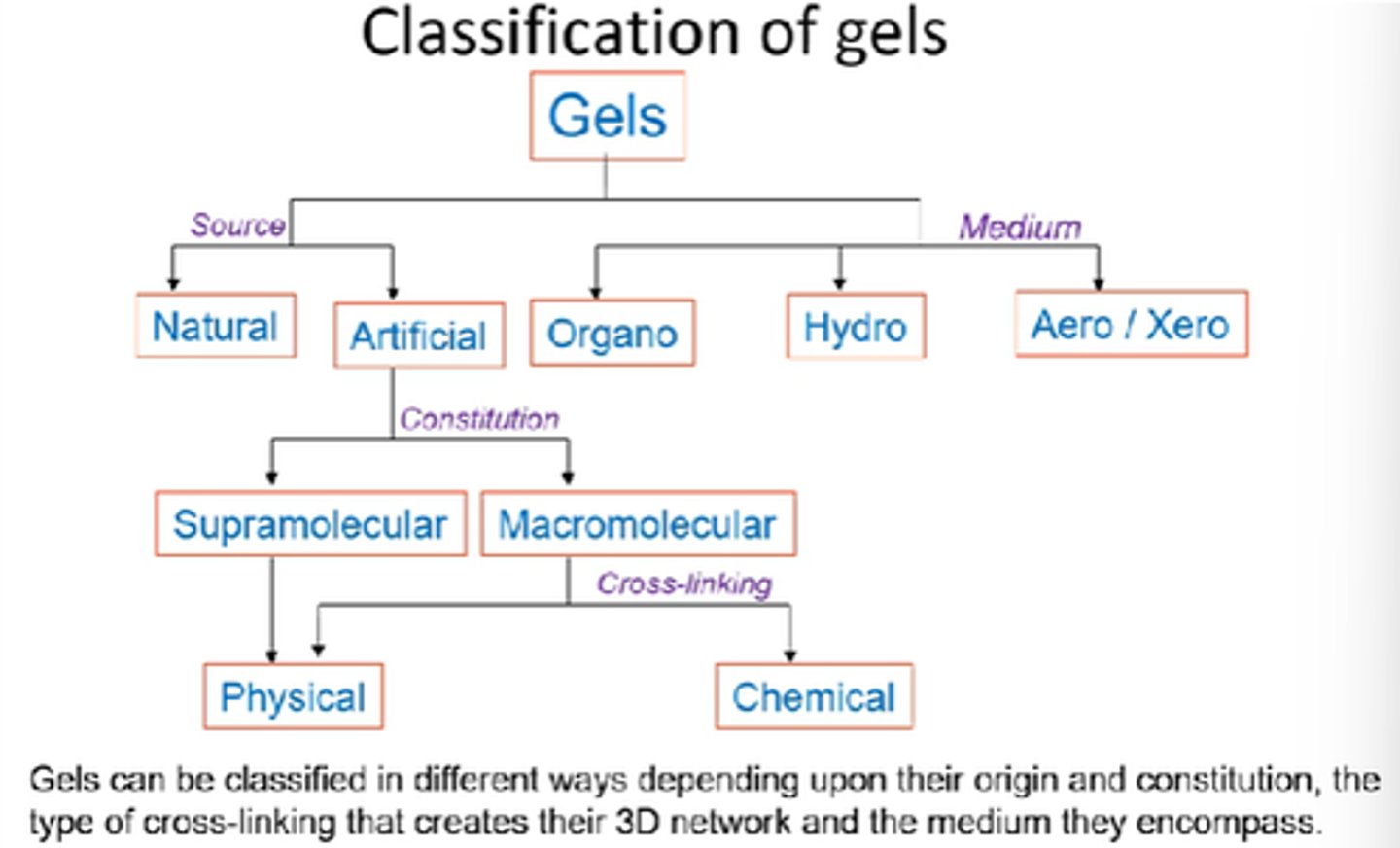
Name the 3x mediums that gels can be classified into.
Organo, hydro, aero.
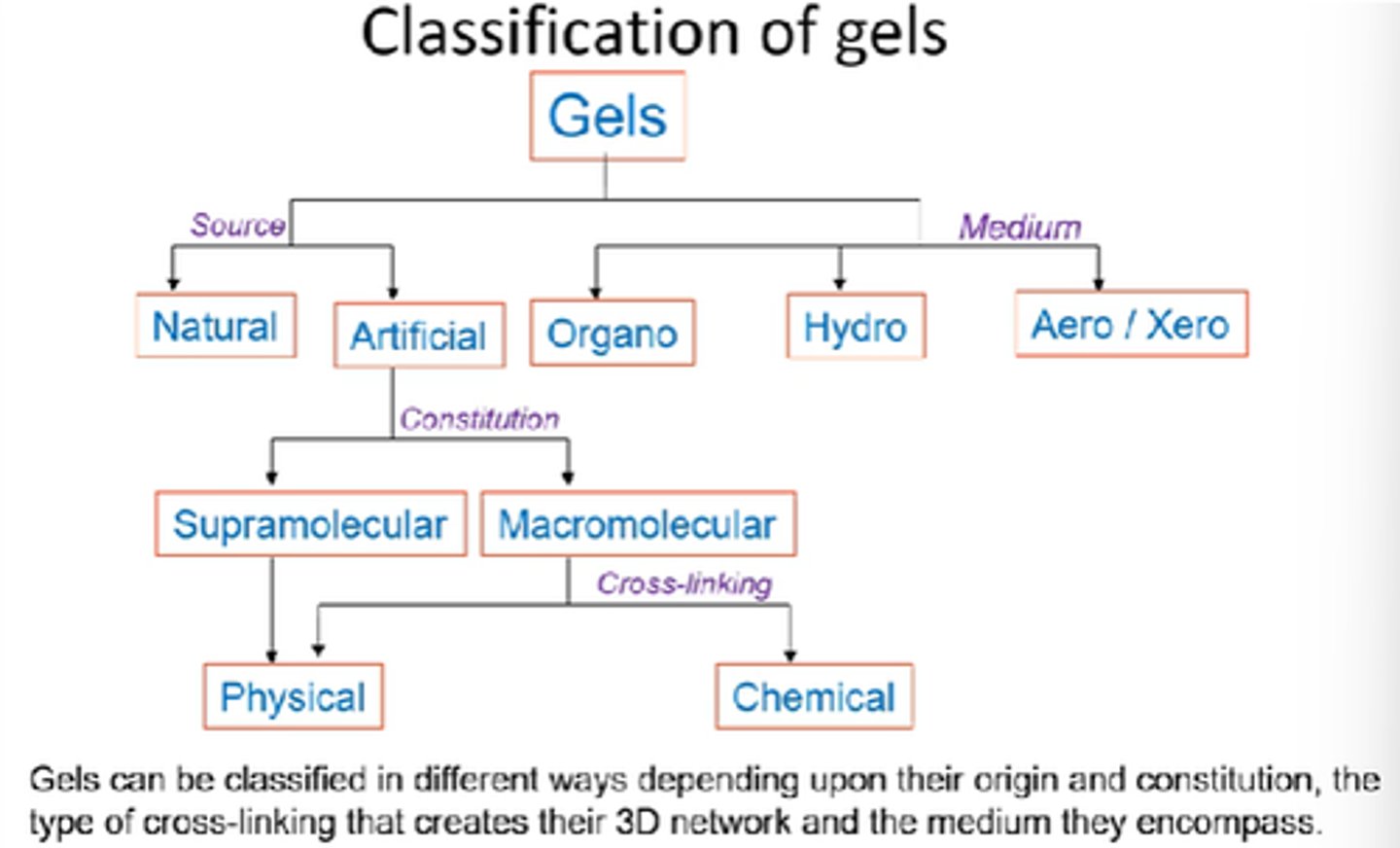
How can gels be classified into sources?
Natural vs artificial
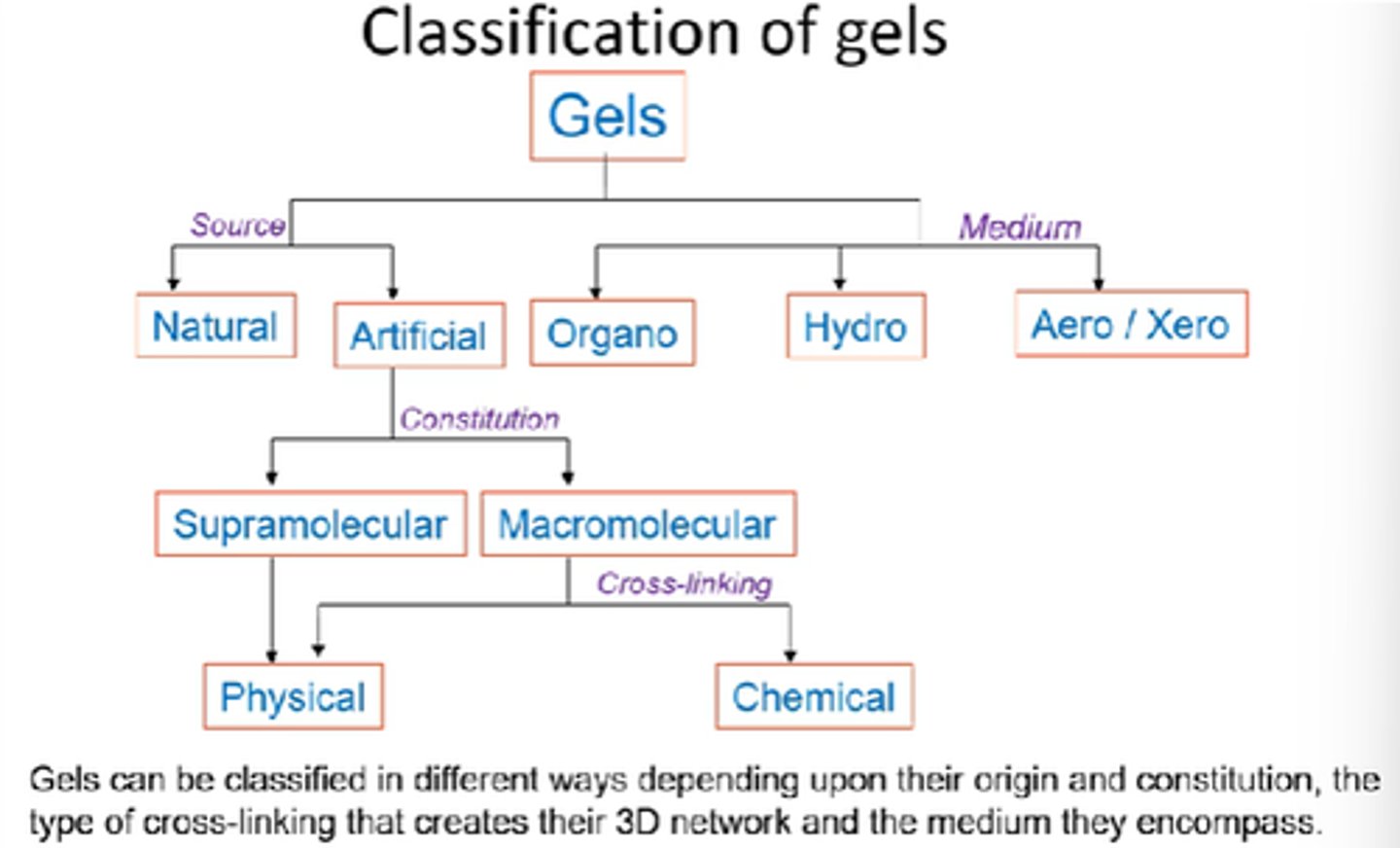
How can artificial gels be further classified?
Supramolecular vs Macromolecular.
Physical vs chemical.
What does hydrogel mean?
Solvent = water.

What does organogel mean?
Solvent = organic liquid.

What are most naturally occurring gelators classified as?
How do they form gels?
Macromoleculars!
Form gels by physical cross-linking.
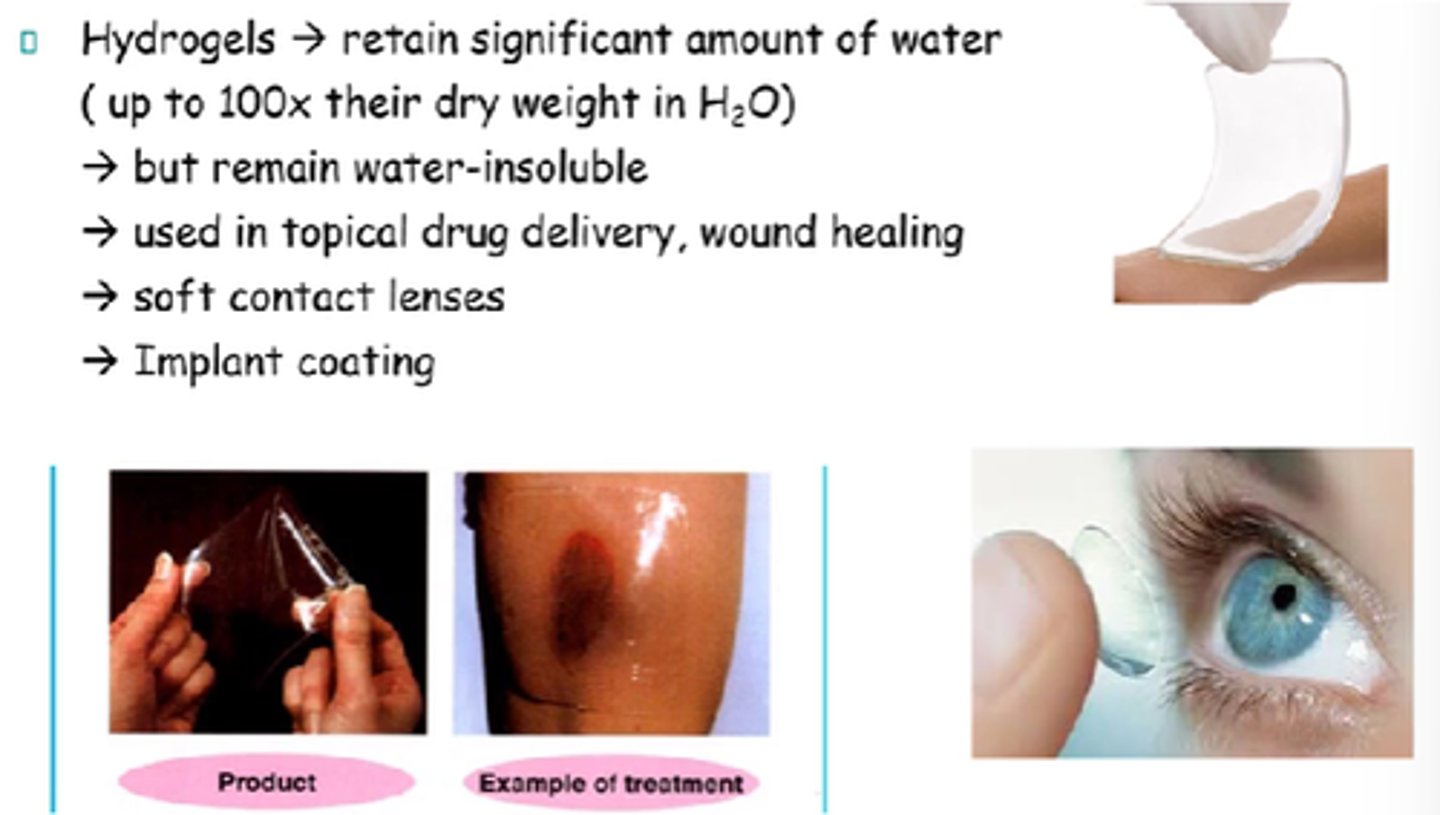
Hydrogels retain up to 100x their dry weight in water, but remain water-insoluble.
What are some uses of hydrogels?
- Wound healing.
- Soft contact lenses.
- Topical drug del.
- Implant coating.
What does diffusion rate of a drug in hydrogel depend on?
Physical structure of polymer network and its chemical nature
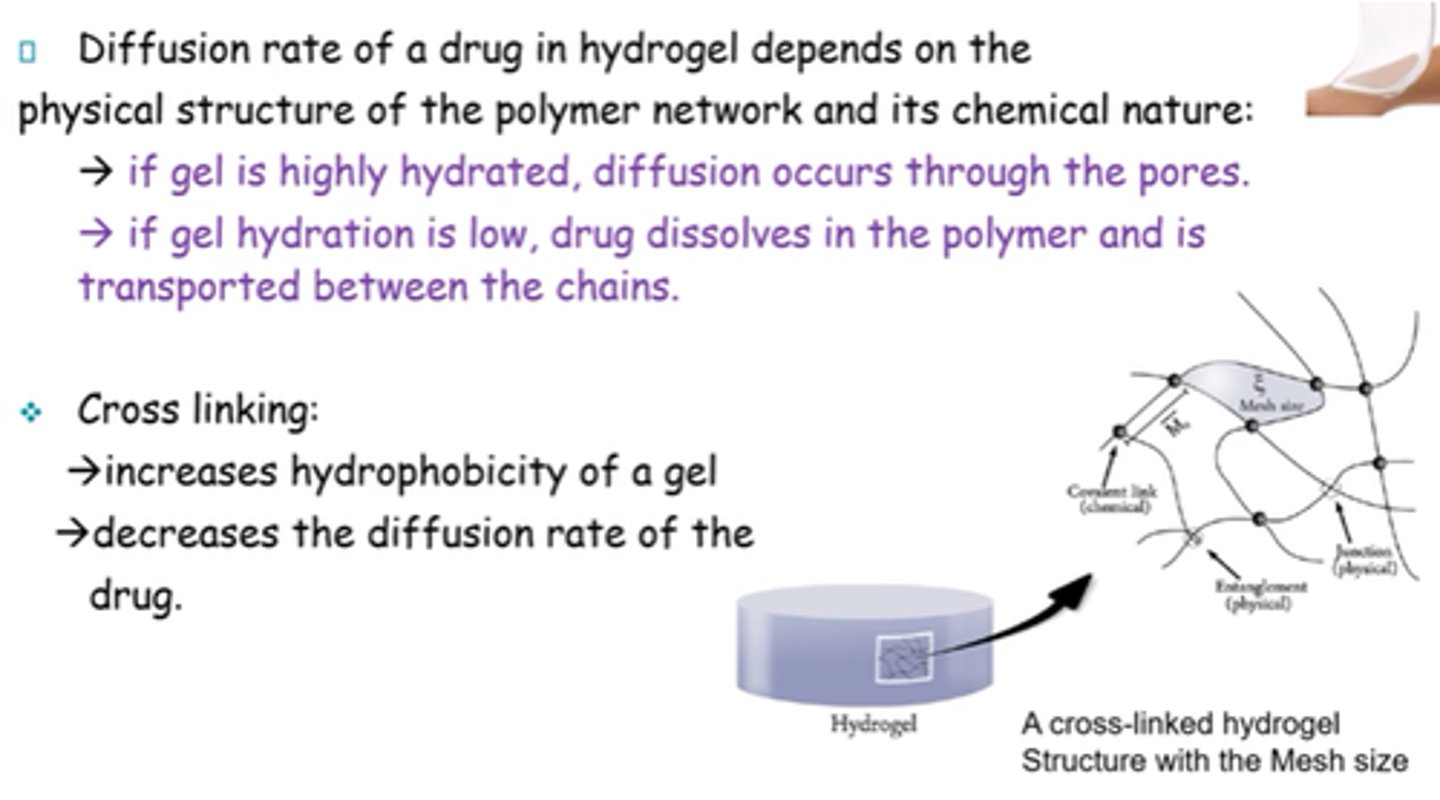
What happens to the drug if the hydrogel is highly hydrated?
Diffusion occurs through pores
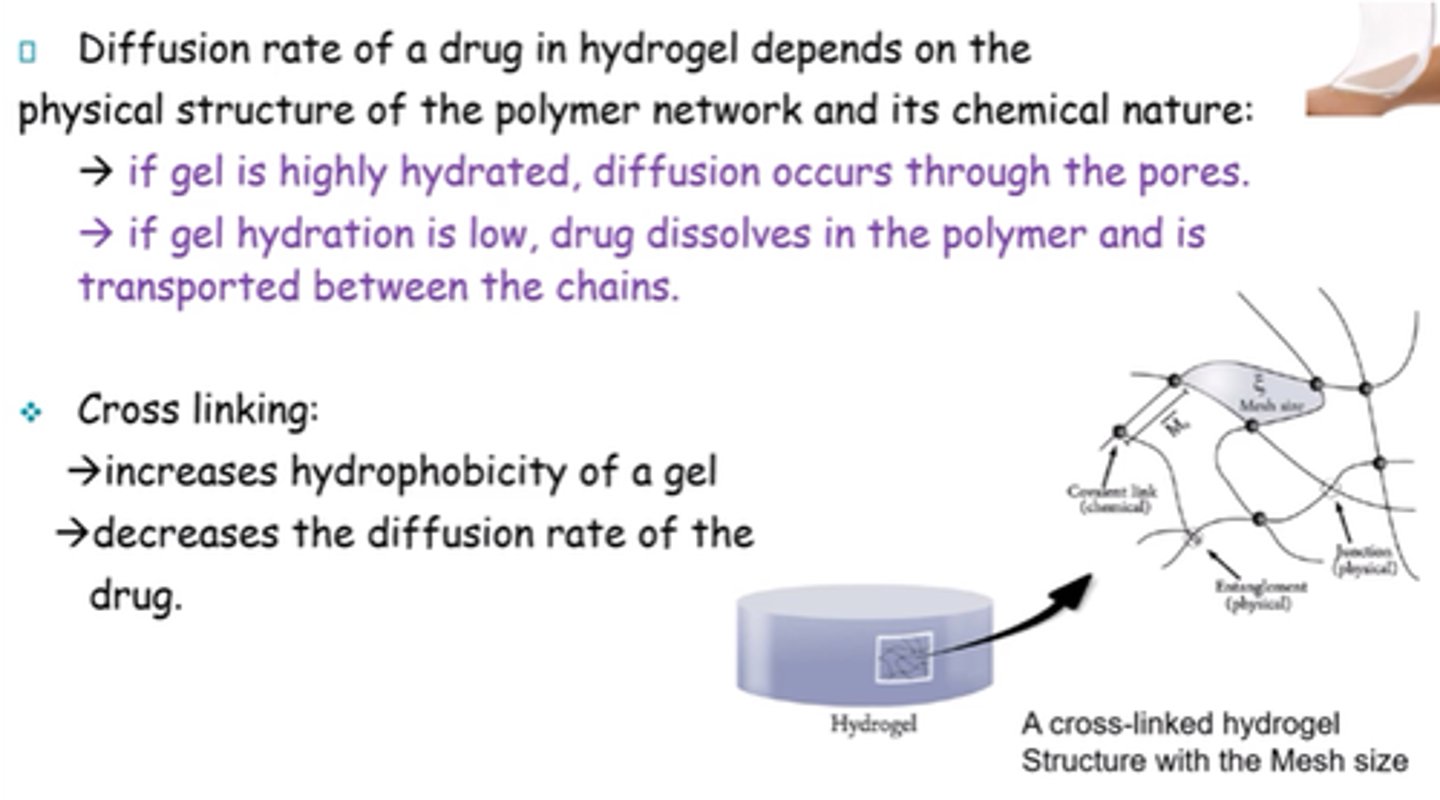
What happens to the drug if hydration of the hydrogel is low?
Drug dissolves in the polymer and is transported between chains.
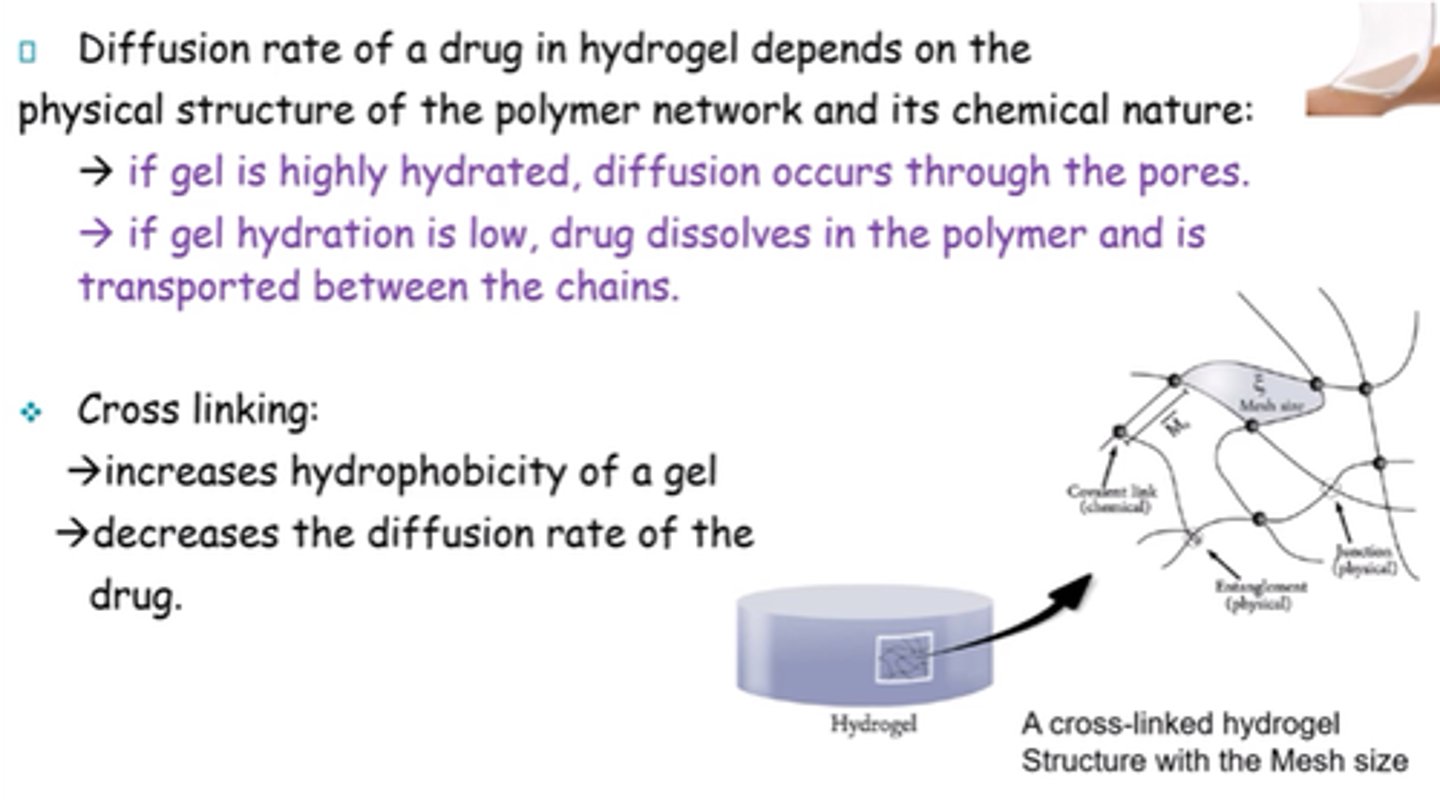
What is the result of cross-linking a gel?
- Increases hydrophobicity.
- Decreases diffusion rate of drug.
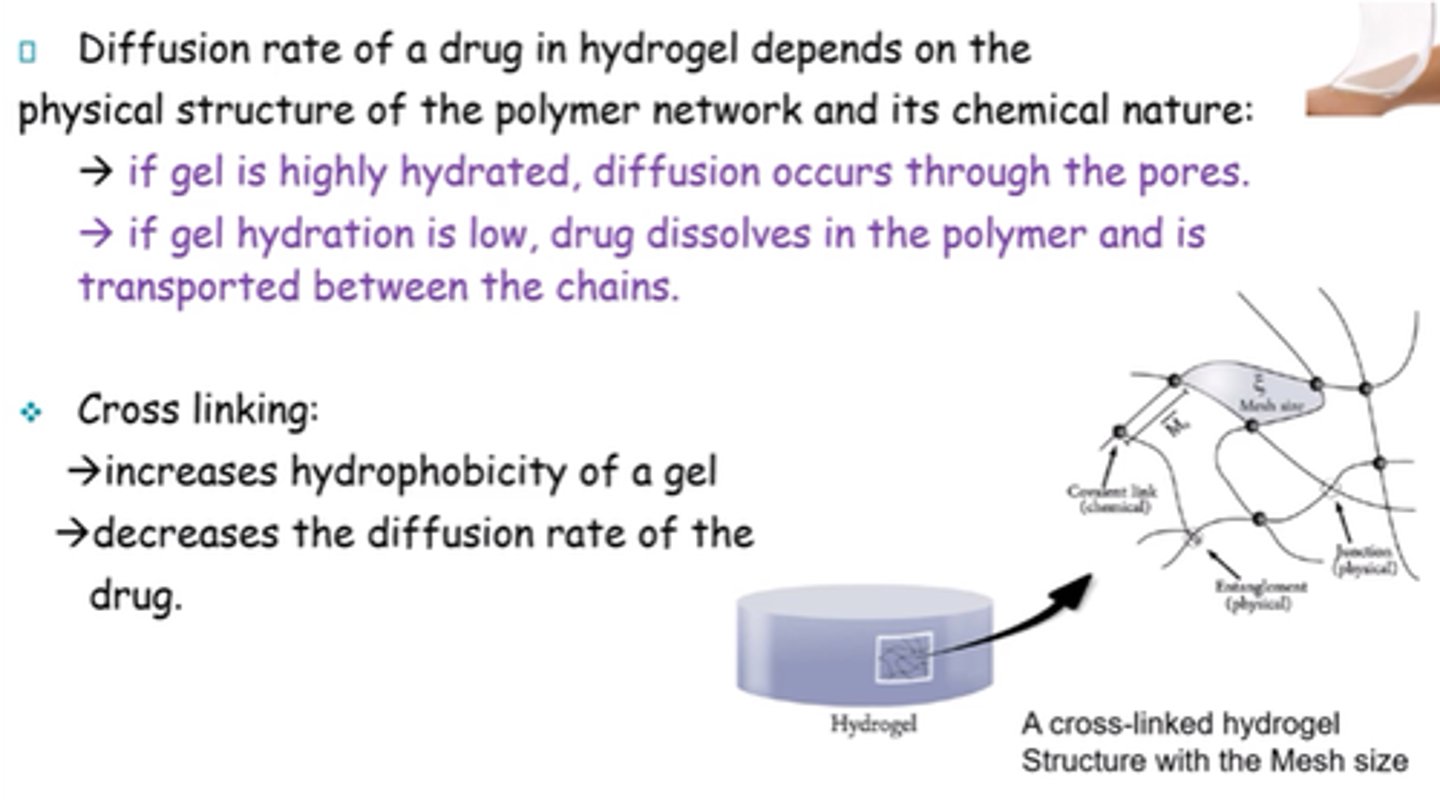
What factors can affect drug release in hydrogels?
Heat, pH or electric current.
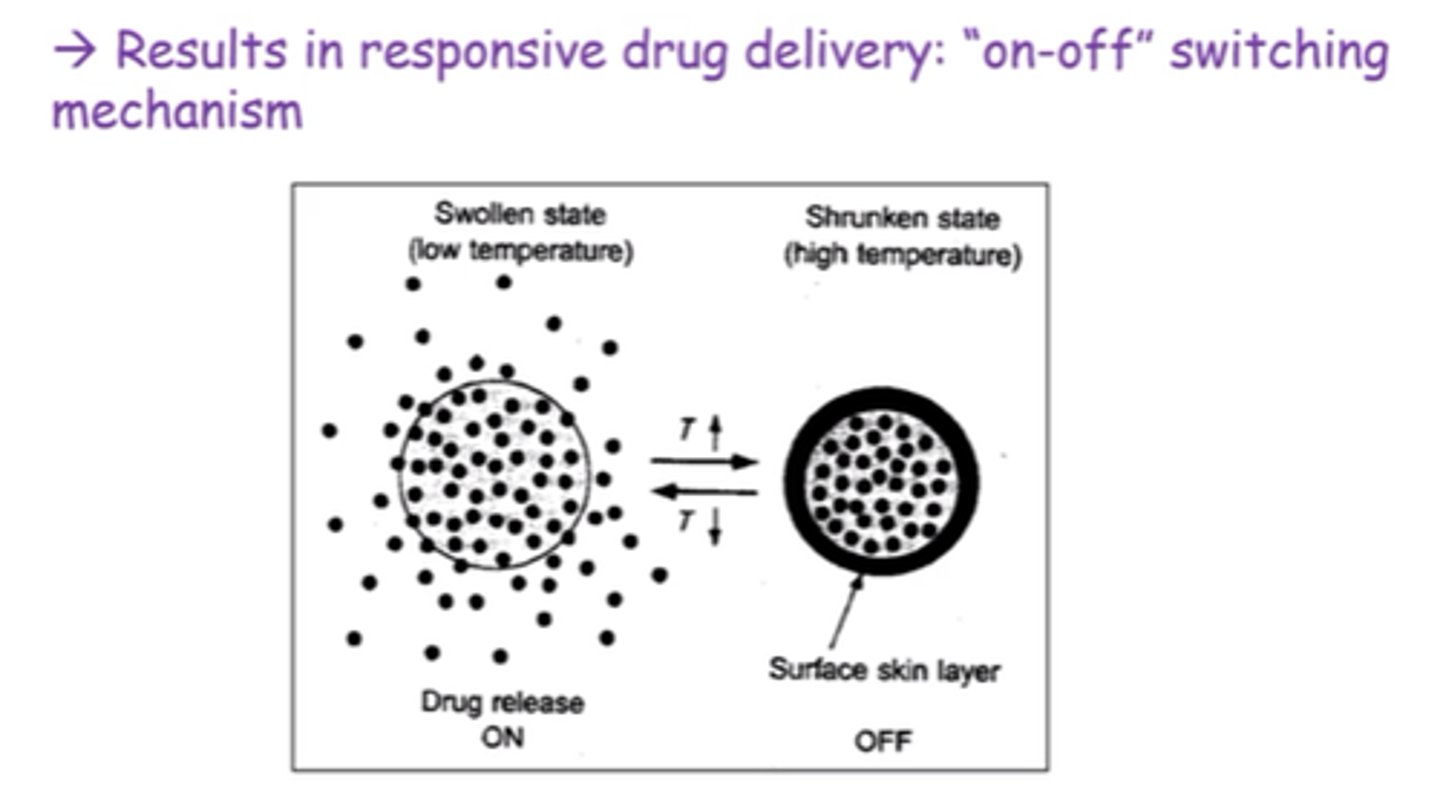
What's the difference between gels formed by strong chemical bonds, and weak non-covalent interactions?
Strong chemical bonds -> can't be dissolved and thermally irreversible.
Non-covalent -> reversible
What's the main difference between Type I and Type II gels?
Type I -> irreversible systems.
Type II -> heat-reversible.
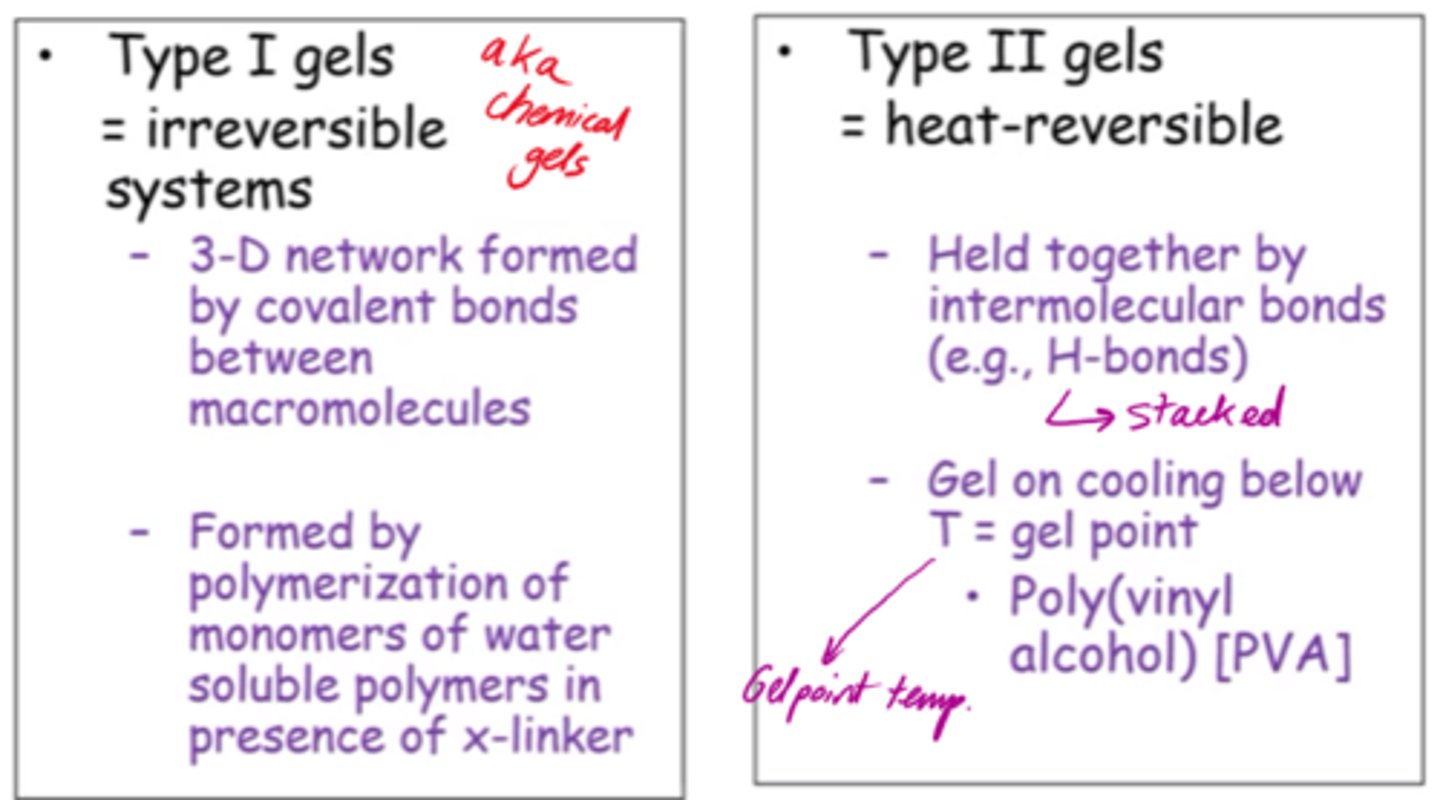
What are Type I gels, aka chemical gels?
- Irreversible systems.
- 3D network formed by covalent bonds between macromolecules.
- Formed by water soluble polymers in presence of cross-linker
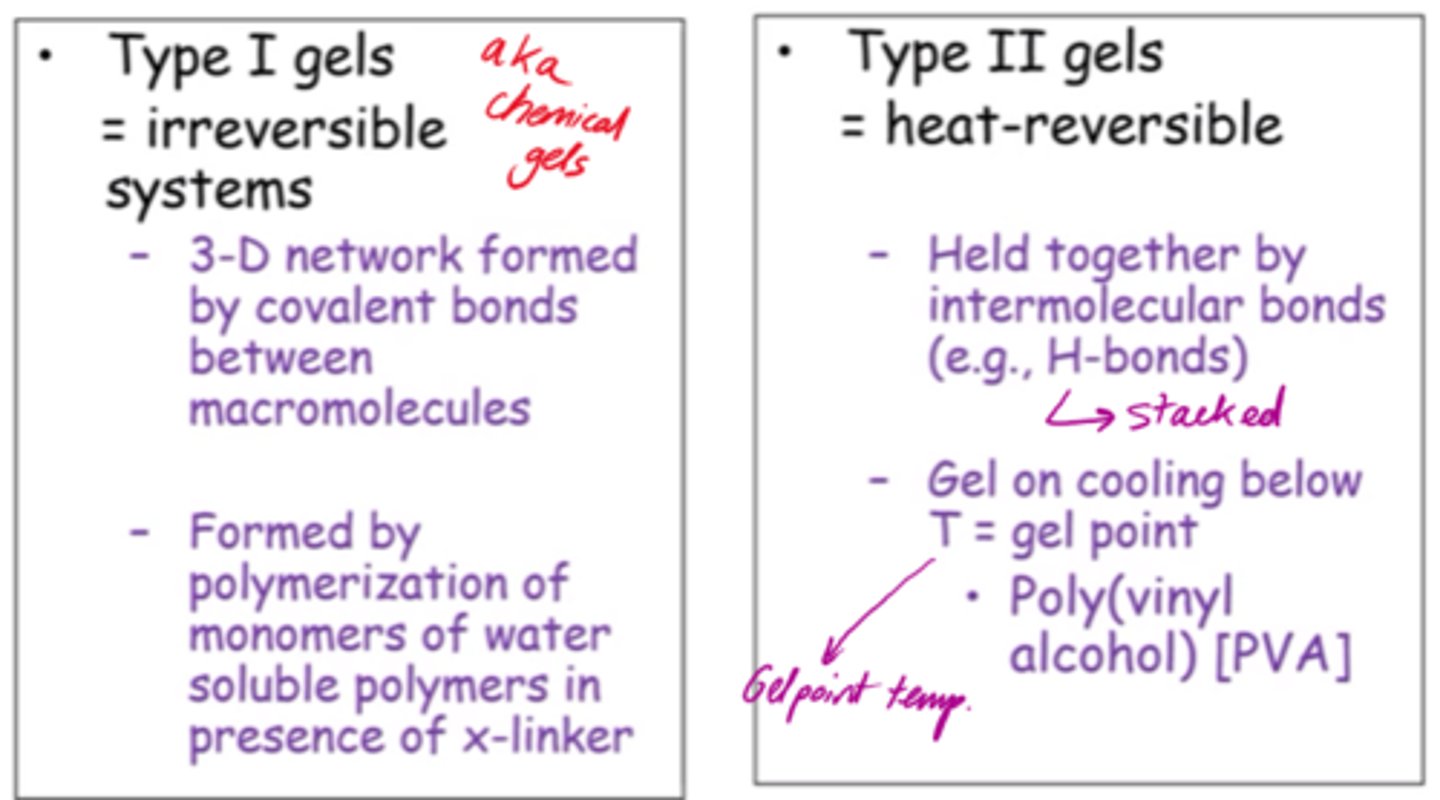
What are Type II gels?
- Heat reversible.
- Held by intermolecular bonds eg stacked H bonds.
What are Type II PVA solutions?
- Viscous in water.
- Applied topically to skin.
- Dried rapidly, leaving plastic film w/drug in contact w/skin. Releases drug accordingly.
What happens if water-soluble polymer chains are covalently cross-linked into a 3D structure?
- Gel forms when dry material interacts w/water.
- Polymer swells but can't dissolve due to cross-links.

What are SAFINs?
Self-Assembled Fibrillar Networks.
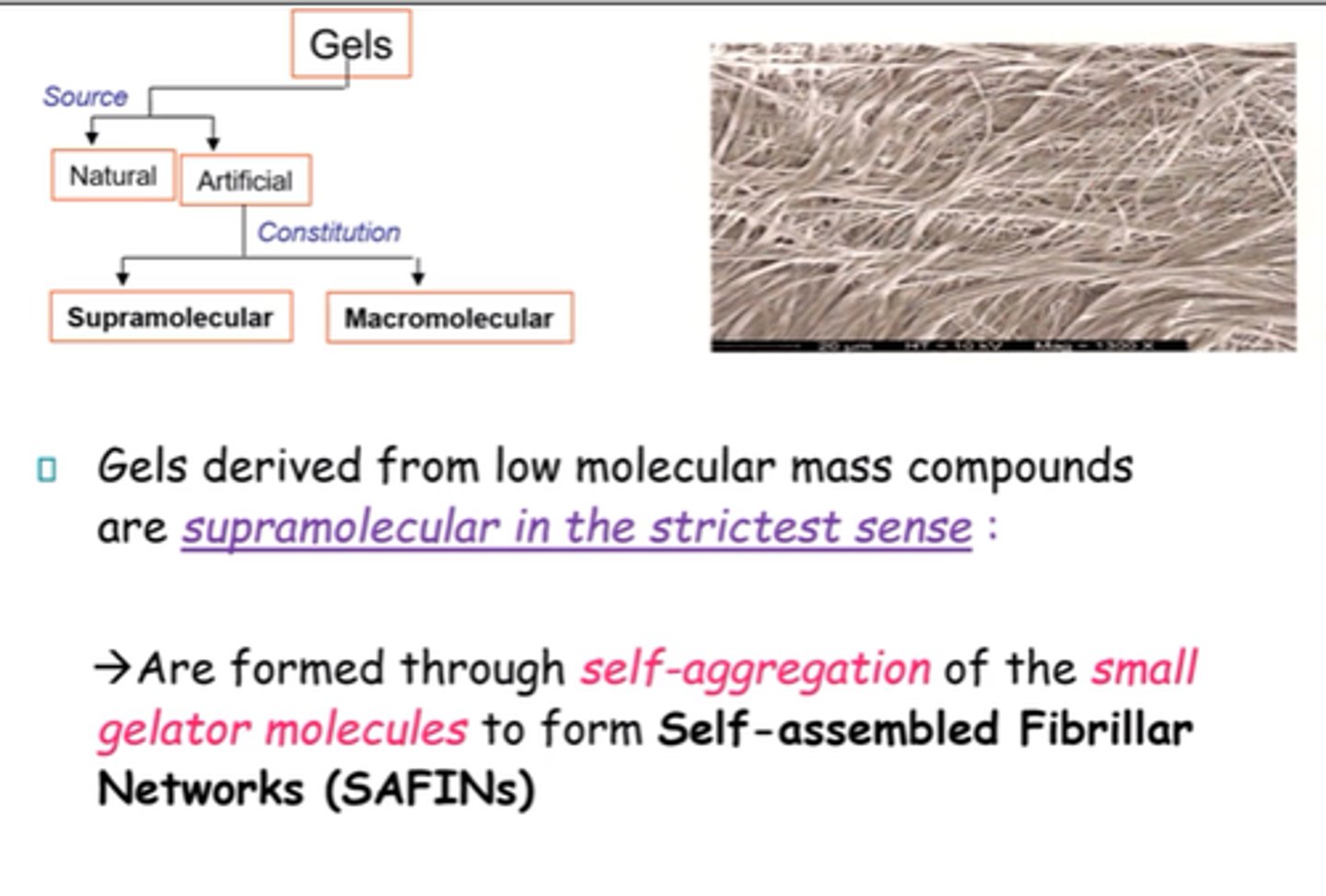
How are SAFINs formed?
- Through self-aggregation of small gelator molecules.
- Non-covalent interactions and VdWs.

Why are SAFINs thermally reversible?
- Networks involve weak interactions.
- Can be readily transformed to a fluid by heating.

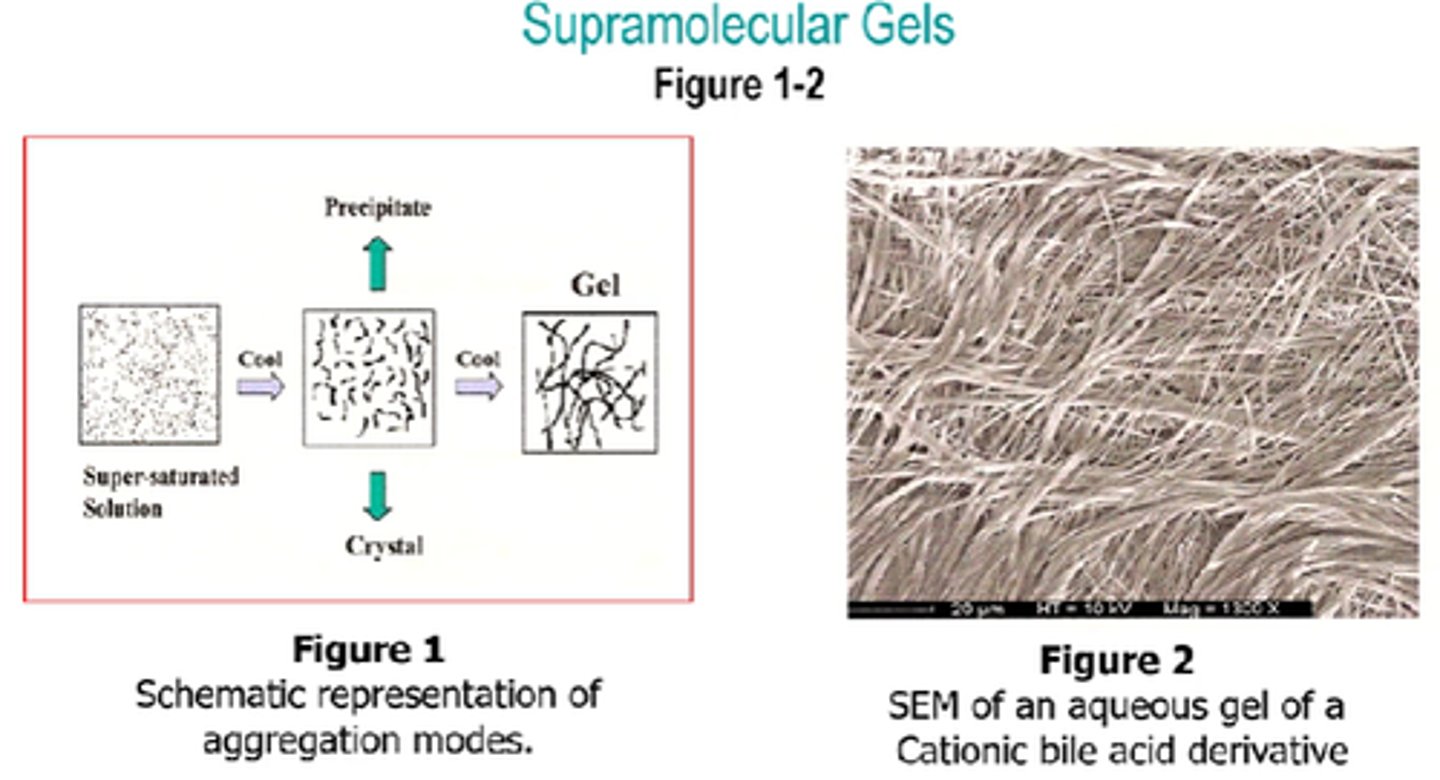
How are Supramolecular gels of a low Mw compound prepared?
- Heat gelator in solvent.
- Cool the resulting isotropic supersaturated solution to room temp.

When making supramolecular gels, what happens when the hot solution is cooled?
Molecules condense and either:
1 - crystallise.
2 - randomly aggregate into an amorphour precipitate.
3 - An intermediate aggregation between (1) and (2) gives a gel.
Give some applications of supramolecular gels:
- Cosmetics.
- Media for tissue engineering.
- Controlled release in drug delivery.
- Wound dressing.
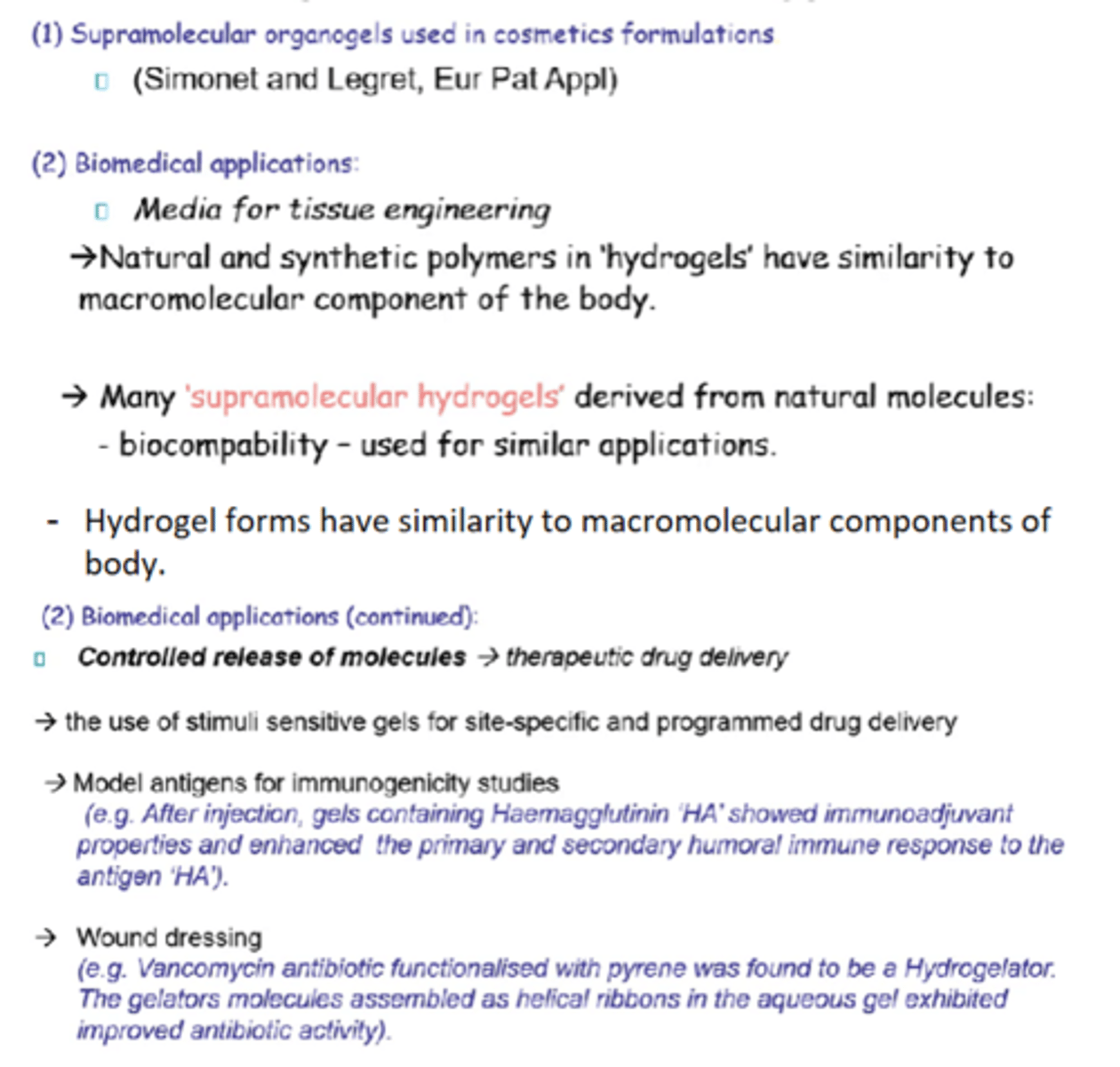
What is micellisation?
The self-assembly of amphiphilic molecules in water into micelles when surfactant conc exceeds CMC.
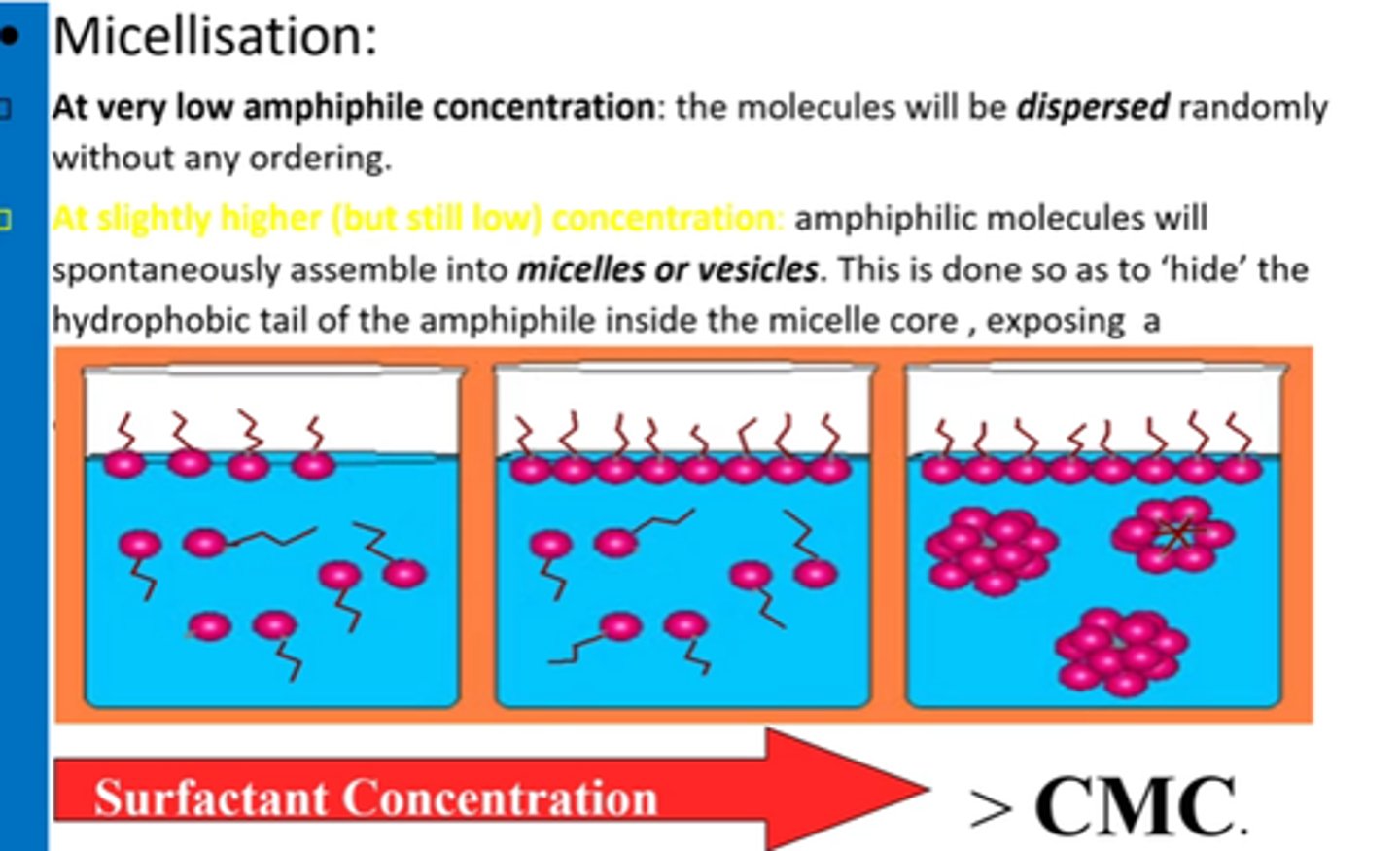
What is CMC?
Minimum conc of surfactant required for micelles to start forming.
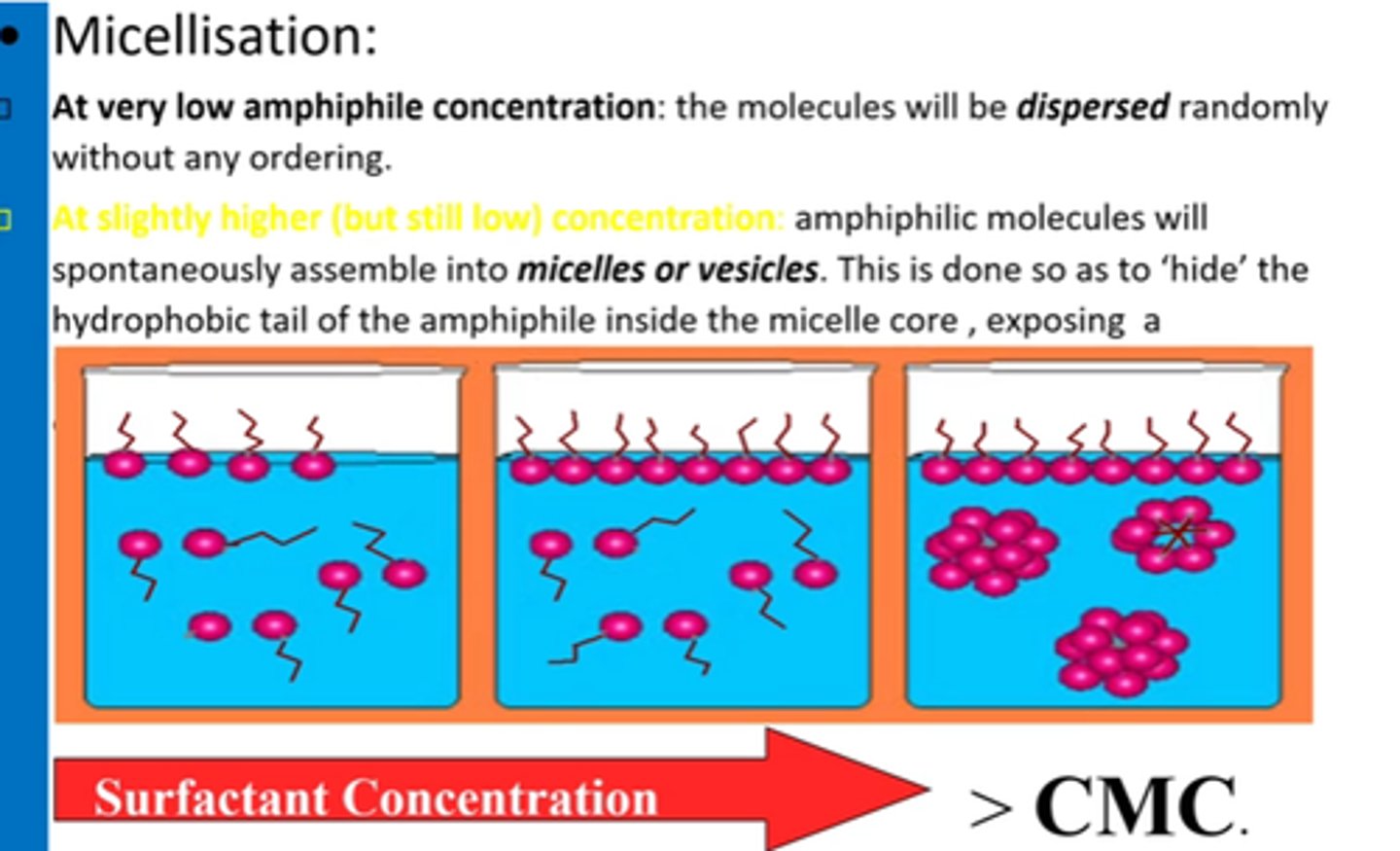
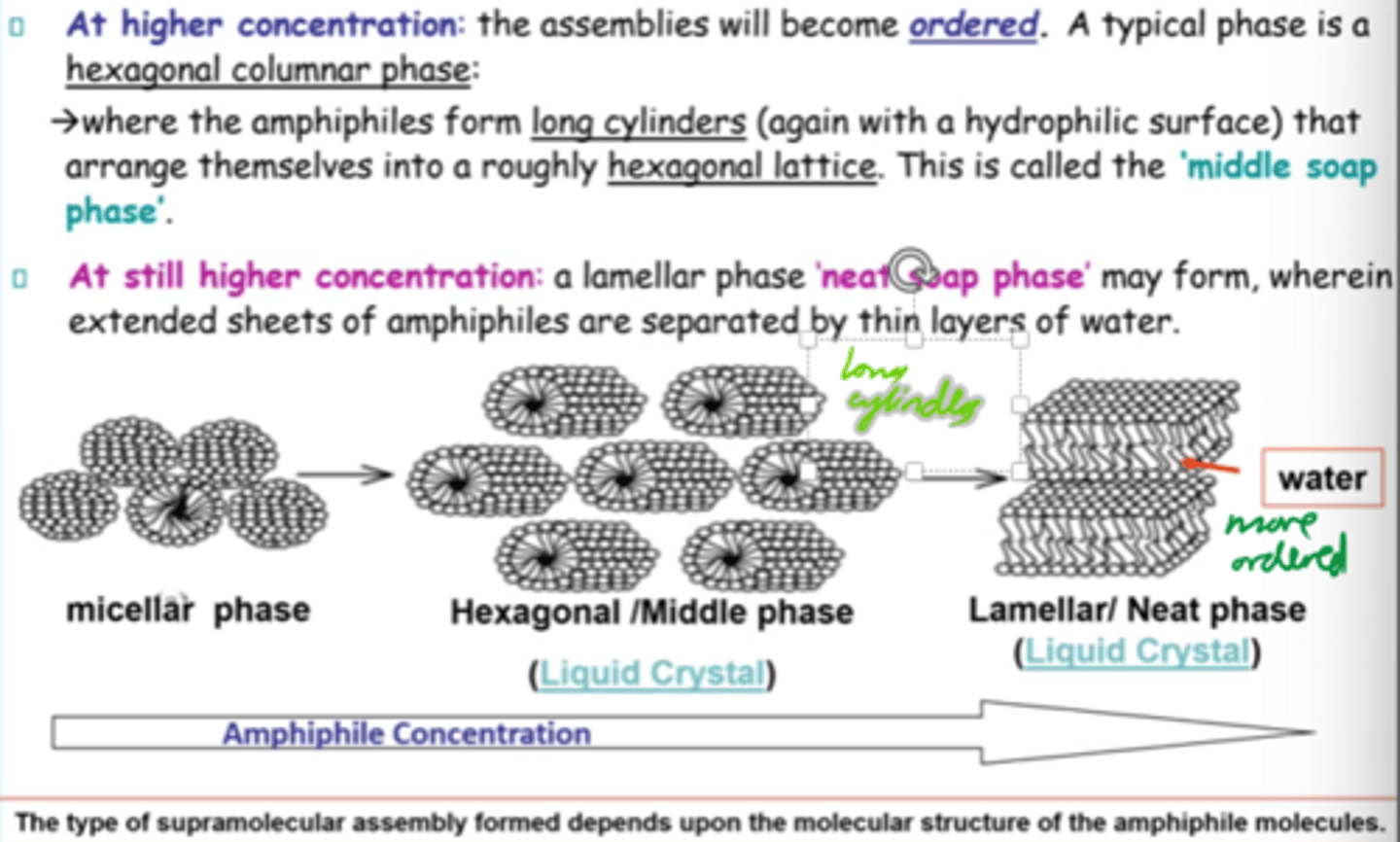
At higher amphiphile concentrations, what structure forms?
Hexagonal columnar phase:
Amphiphiles (surfactant molecules) arrange into long cylinders in a hexagonal pattern
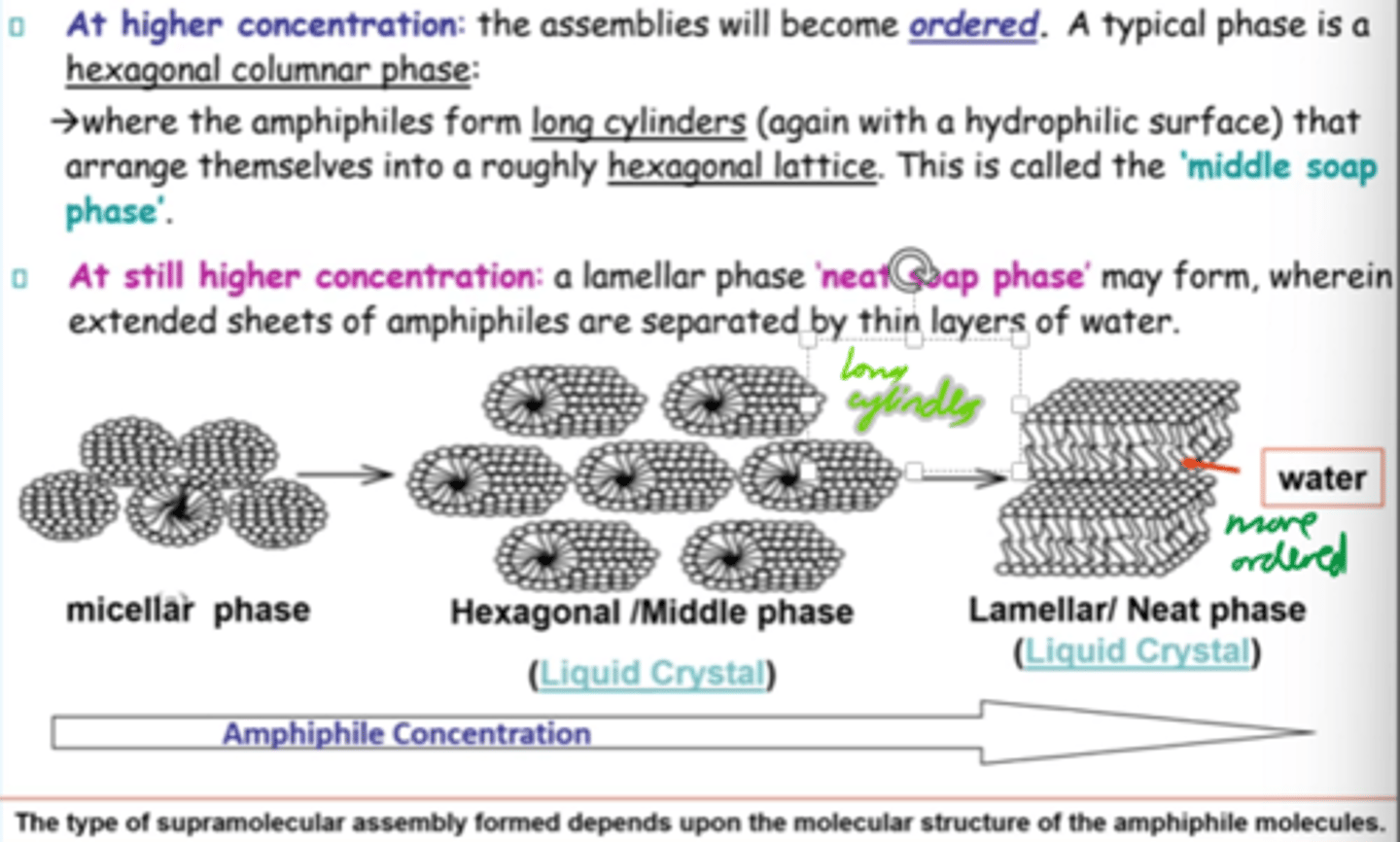
What is the "middle soap phase"?
Hexagonal phase where cylindrical amphiphiles form a roughly hexagonal lattice.
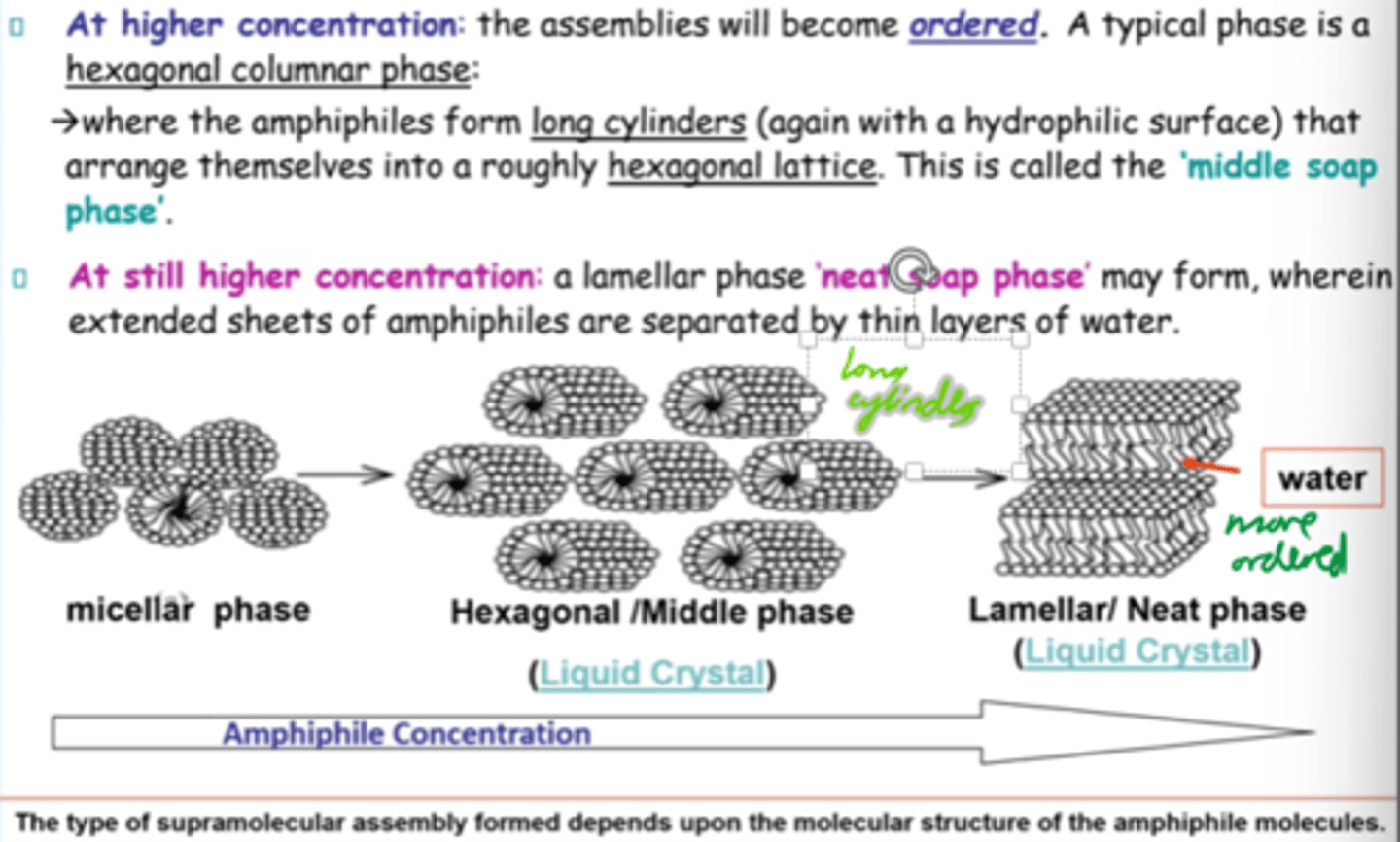
How does amphiphilic assembly change w/conc?
Micellar -> hexagonal -> lamellar structures as conc increases.
What are liposomes?
Vesicular structures based on one or more lipid bilayers encapsulating an aqueous core.
Liquid crystals.
What are liposomes mainly composed of?
Phospholipids
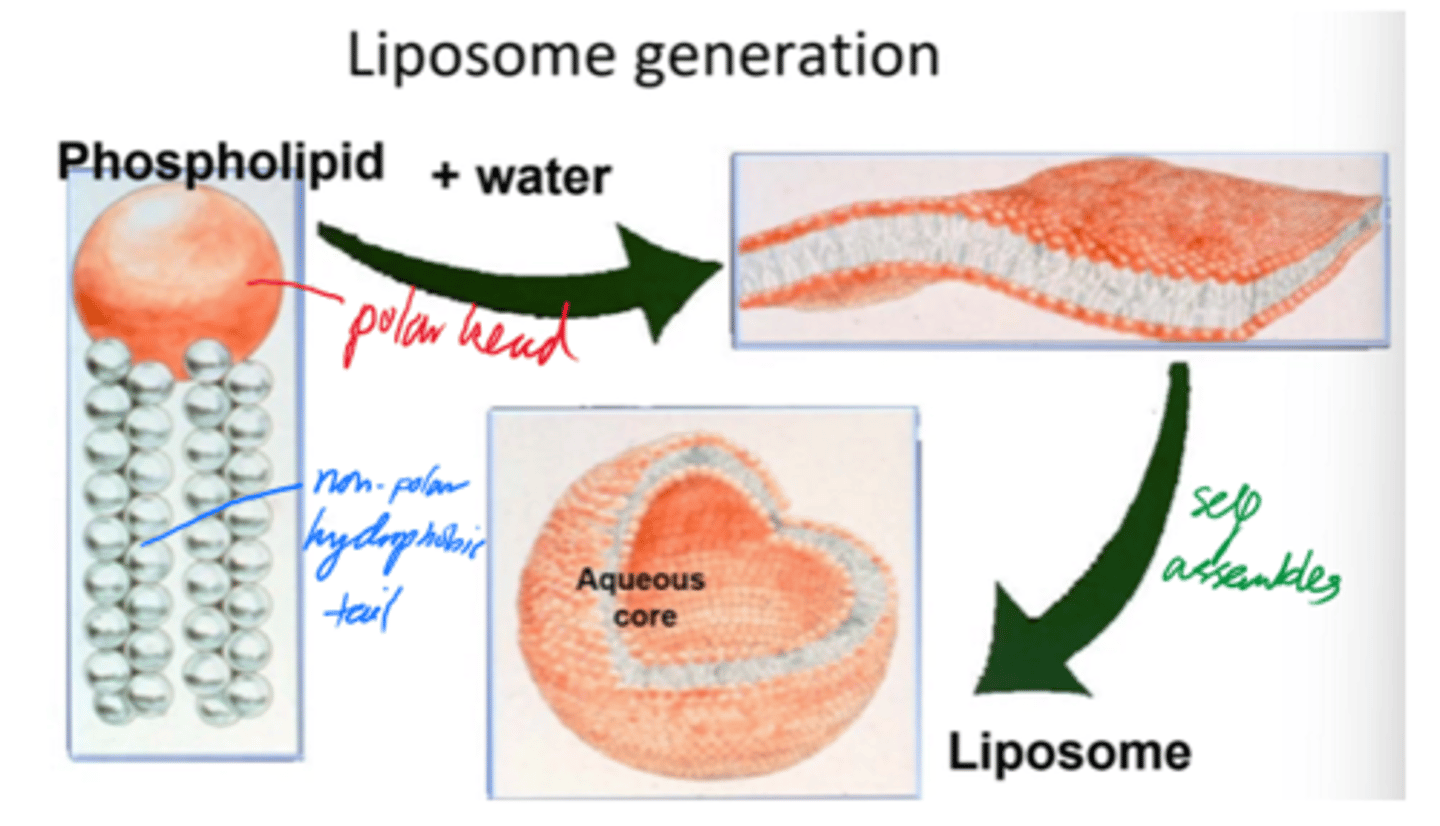
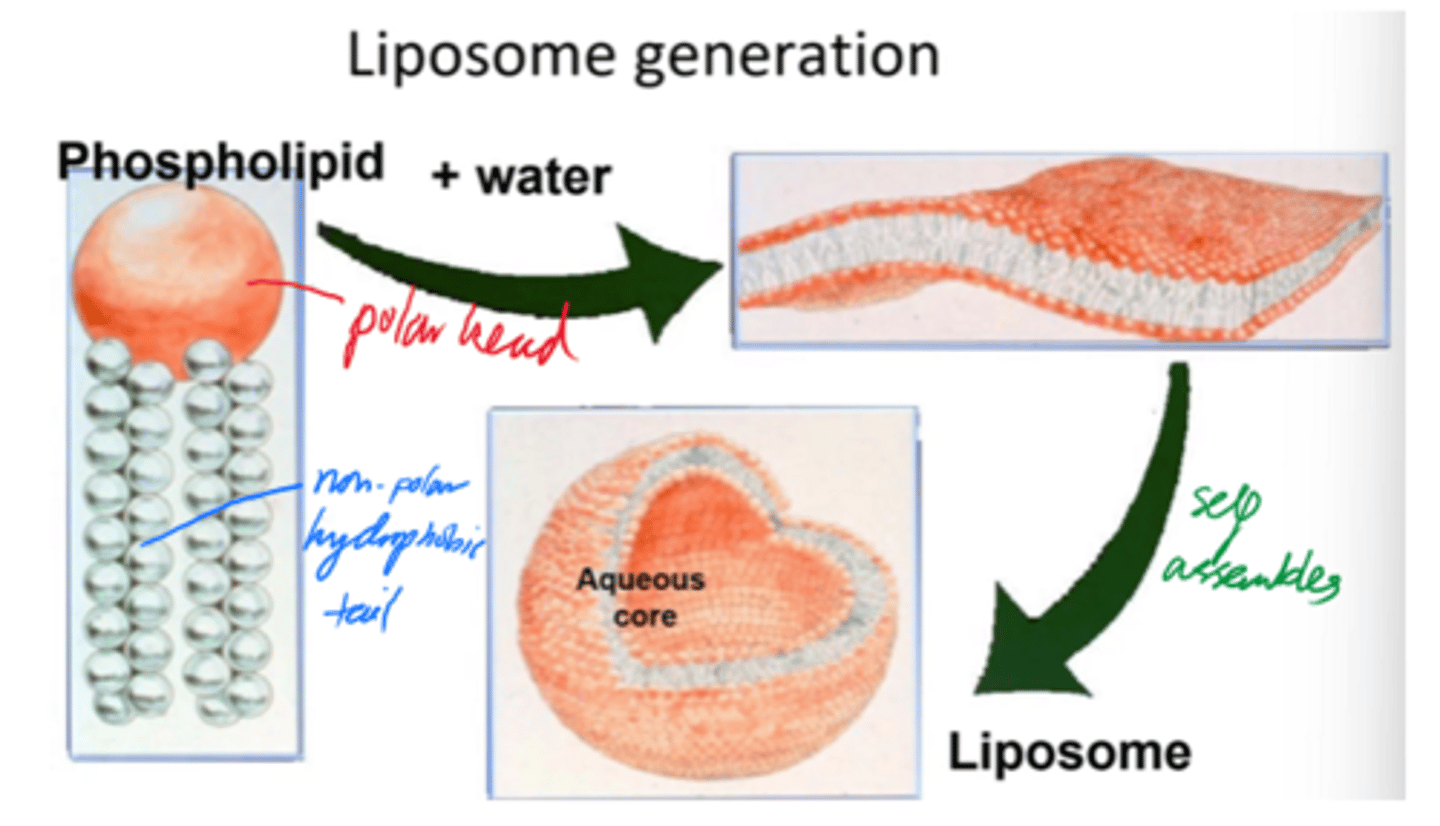
Describe liposome formation.
- Phospholipids self-assemble into bilayers when added to water.
- Bilayers curve + close into spherical liposomes.
- Minimises edge exposure and encloses aq core.
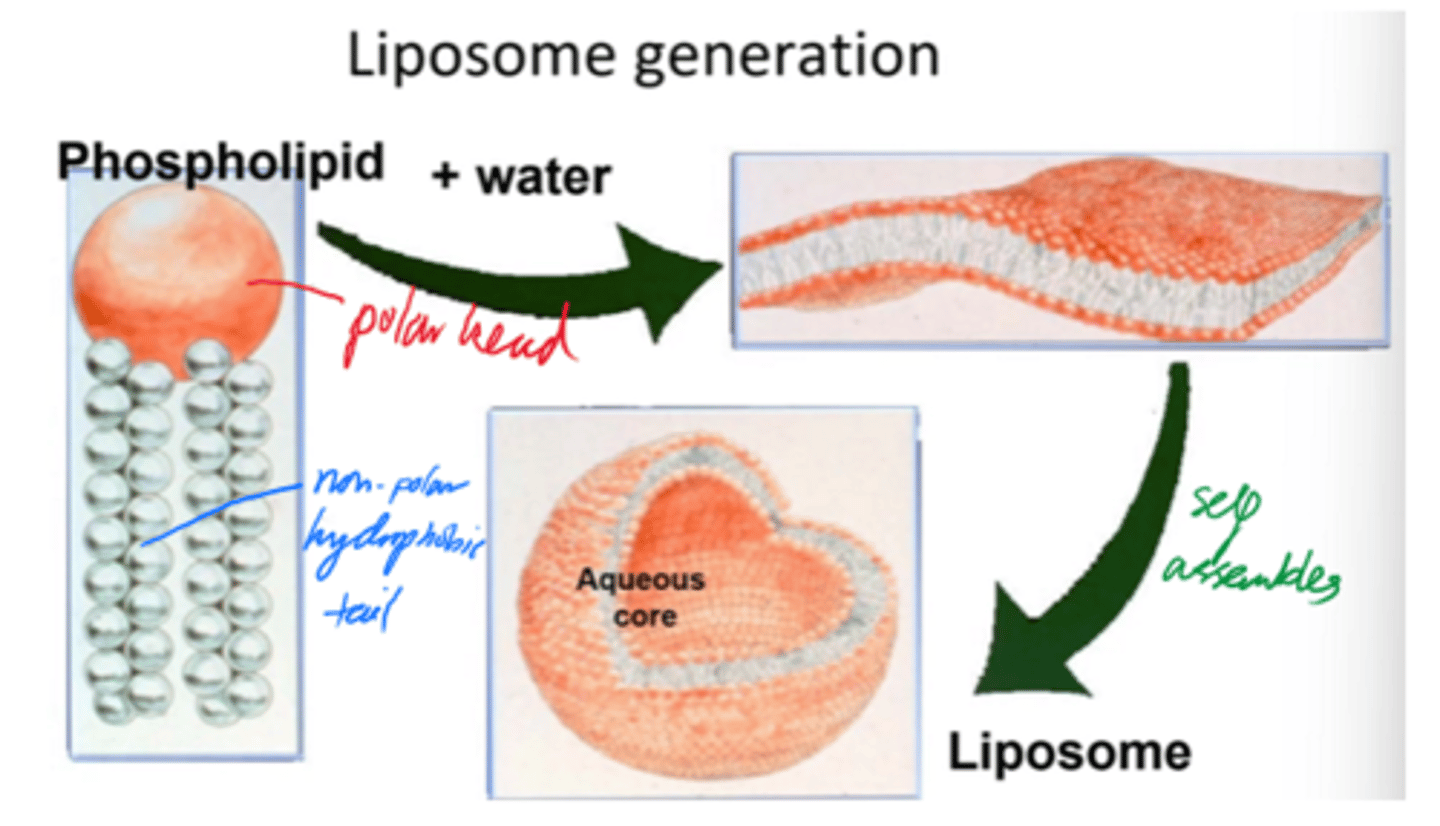
Liposome self-assembly results in a stable, enclosed structure with an internal aq environment.
But what is self-assembly driven by?
Hydrophilic/hydrophobic interactions.
How can liposome structure be described?
Multilamellar or unilamellar.
Eg uni - only 1 bilayer surrounds an aq core.
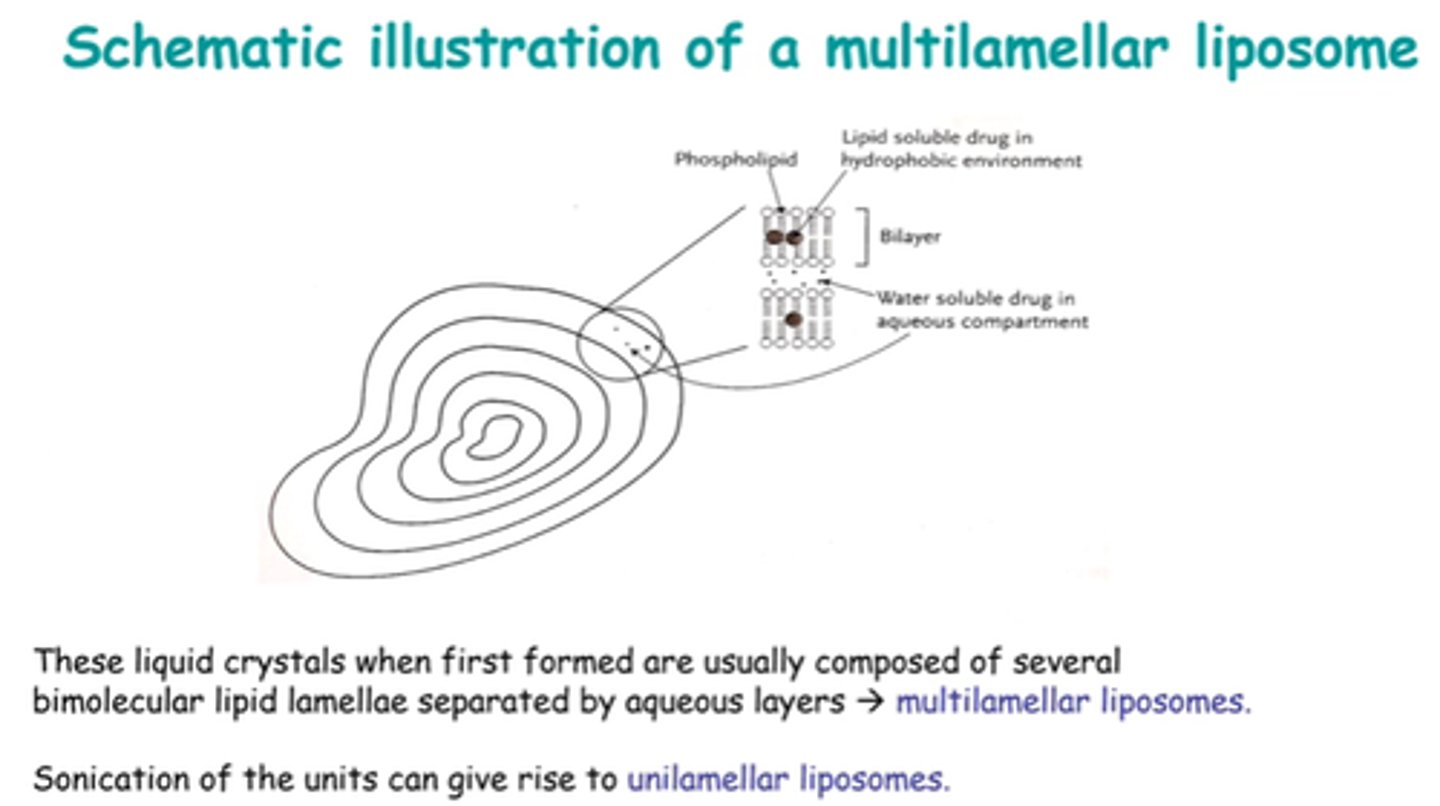
Liposomes are mainly multilamellar when formed.
How can we get a unilamellar bilayer w/single core?
Sonicate!
Why are Liposomes attractive drug carriers?
Can carry highly lipophilic and hydrophilic drugs, AND drugs w/intermediate logP partition.
So carry both water and lipid soluble drugs.
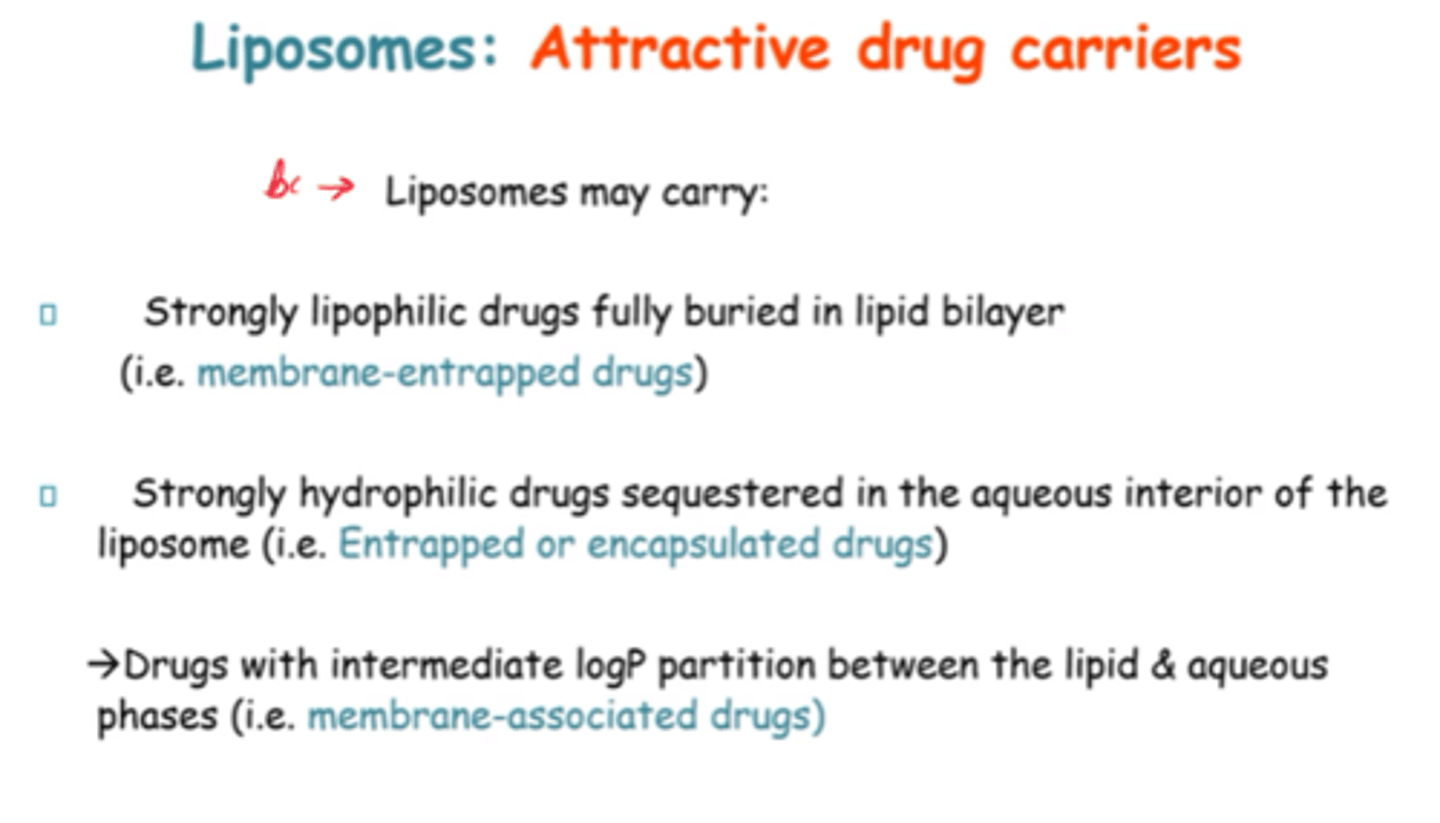
What do the rigidity and permeability of the bilayer depend on?
Type and quality of lipids used.

The presence of what tends to stabilise liposomes? How?
Cholesterol, by rigidifying bilayer.

What does liposome stability depend on?
- Lipid Composition.
- Storage conditions (light, temp, O2).
- Stabilisers
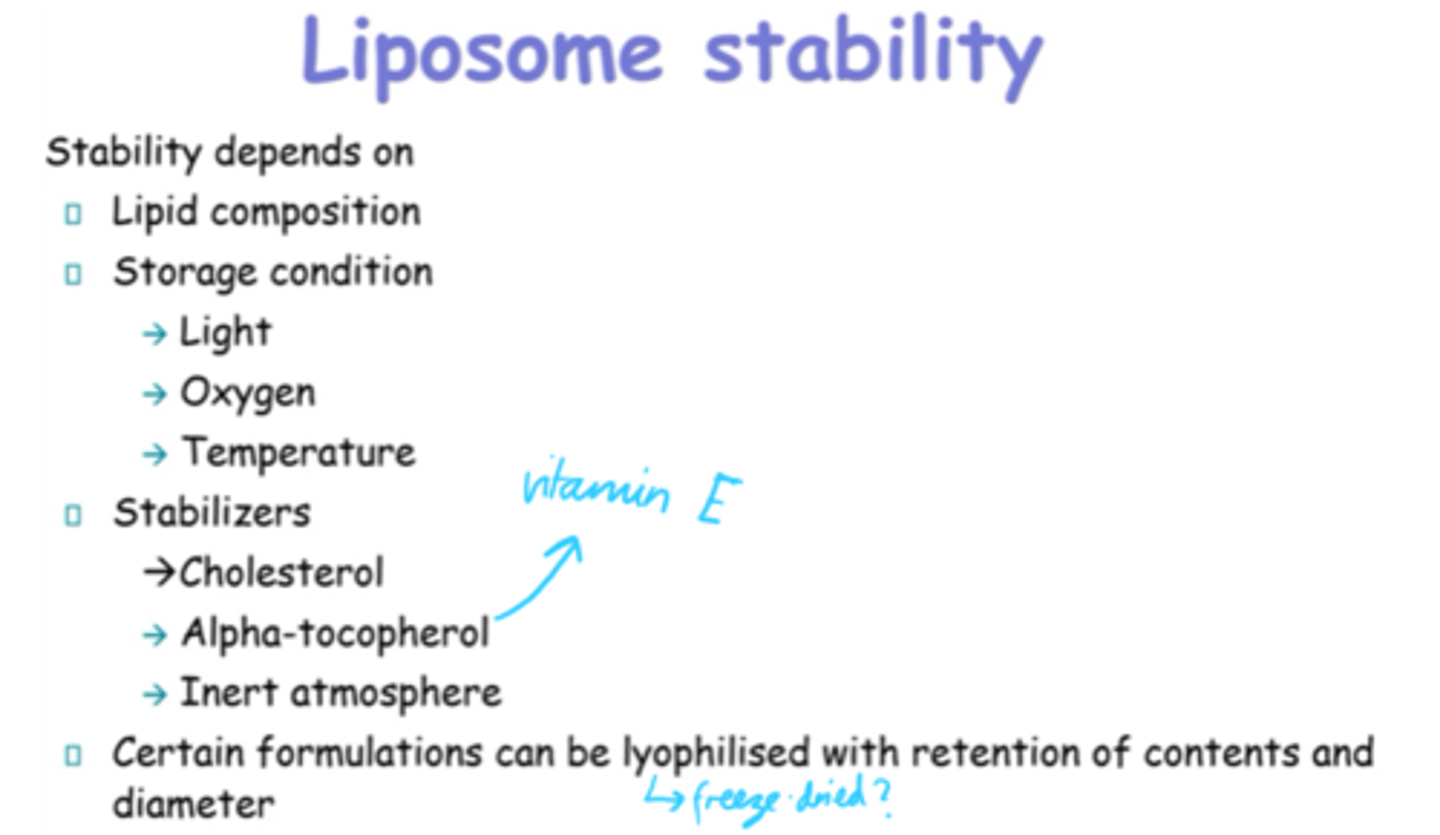
What is Alpha-tocopherol more commonly known as?
Vitamin E
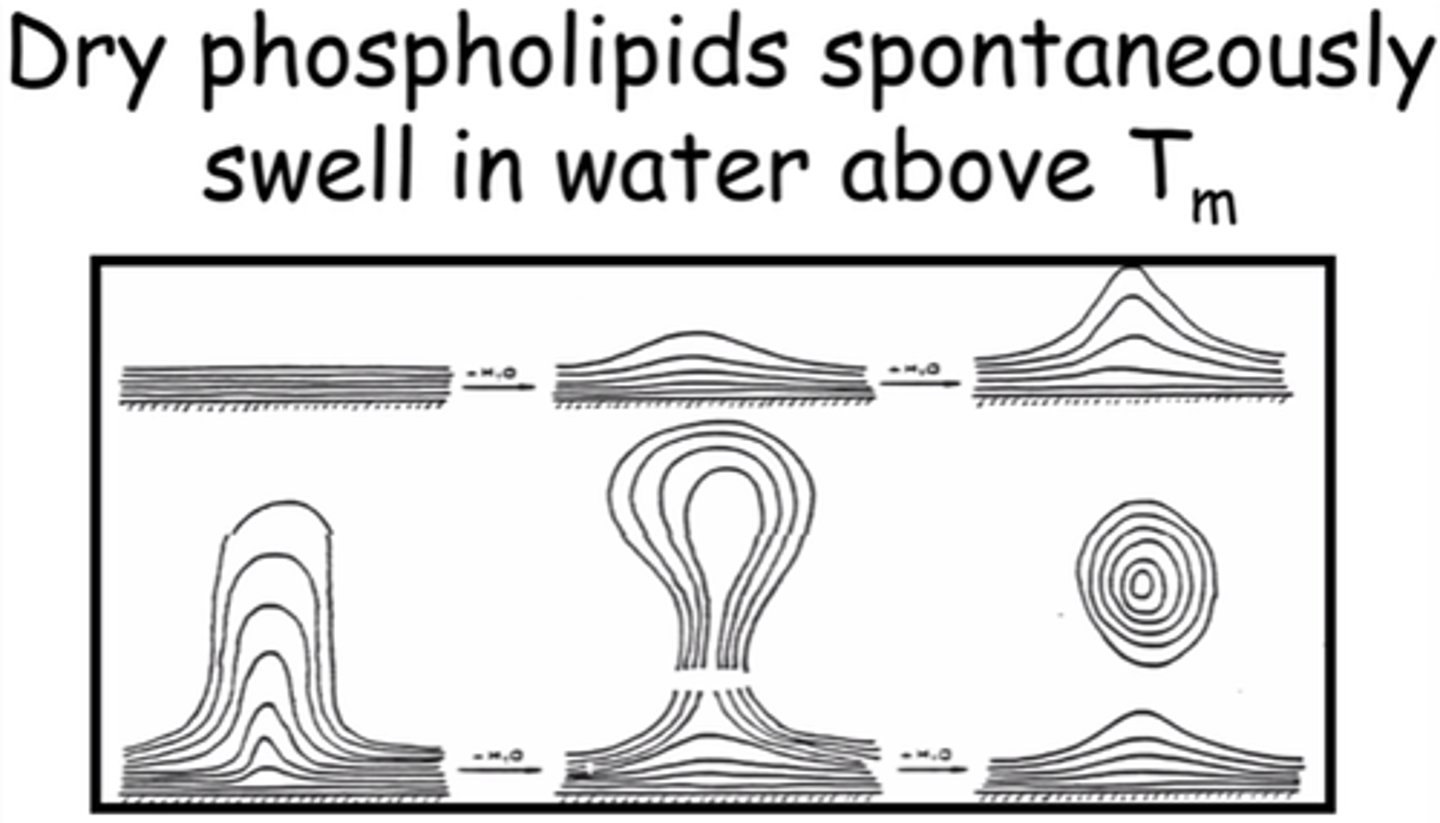
What happens to dry phospholipids in water above Tm?
Spontaneously swell.
What are some advantages to using liposomes as drug carriers?
- Biocompatible and biodegradable.
- Biologically inert.
- Weakly immunogenic.
- Low toxicity.
- Good biodistribution.
- Good for targeting receptors
What are the 4x main classifications of liposomes?
- Conventional.
- Stericly stabilised. (aka stealth)
- Immunoliposomes. (aka antibody targeted)
- Cationic liposomes.
What is the main use of conventional liposomes in drug delivery?
Passive targeting of mononuclear phagocyte system (MPS), esp. liver and spleen
What advantage do conventional liposomes offer to encapsulated drugs?
- Protect drugs from degradation.
- Target tissues w/discontinuous endothelium (eg liver, spleen)
What happens to conventional liposomes after IV admin?
- Taken up by MPS cells.
- Removed from blood circulation via liver and spleen.
What is MPS?
Mononuclear Phagocyte System.
Name a commercial product using conventional liposomes for antifungal therapy.
AmBisome (liposomal amphotericin B)
What are cationic liposomes typically used for?
Delivery of genetic material
What are immunoliposomes typically used for?
Active targeting purposes.
Can be either conventional or sterically stabilised.
What are sterically stabilised "stealth" liposomes typically used for?
To prolong circulation times.
What are conventional liposomes typically composed of?
Phospholipids (neutral or -ve) and/or cholesterol
What's the most popular way to produce long-circulating liposomes?
Covalently attach the hydrophilic polymer PEG to liposome bilayers.
What effect does attaching PEG to the liposome bilayers have?
Creates steric barrier against interactions w/molecular and cellular components in the biological environment.
What do immunoliposomes do?
Have specific antibodies on their surface to enhance target site binding.
What's the primary use of immunoliposomes?
Targeted delivery of anti-cancer agents
By which process can immunoliposomes be made long-circulating?
PEGylation
What can be attached to PEGylated liposomes for targeting?
Antibodies
What's a disadvantage is these antibodies are coupled directly to liposome surface?
PEG chains may block or hinder antigen binding due to steric hindrance.
What are the 2x main coupling strategies for attaching antibodies to liposomes?
- Direct coupling to liposome surface.
- Coupling to the terminal ends of PEG chains.
Which is the more favoured coupling strategy for attaching antibodies to liposomes? Why?
Coupling to terminal ends bc:
- Better antigen access.
- Reduced steric hindrance.
Why is the +ve charge of cationic liposomes useful?
Allows them to neutralise -ve DNA, aiding in gene delivery
How do cationic liposomes help DNA delicery?
They condense DNA into a compact structure by neutralising its -ve charge, facilitating its encapsulation and delivery.
What is a potential side effect of cationic liposomes via IV route?
They can activate the complement system and cause ADRs.