Biochemistry 12U
1/103
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
104 Terms
7 functional group names
Methyl, Hydroxyl, Carbonyl, Carboxyl, Amino, Sulfhydryl, Phosphate groups
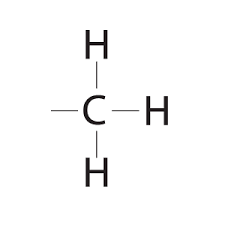
Methyl group
CH3
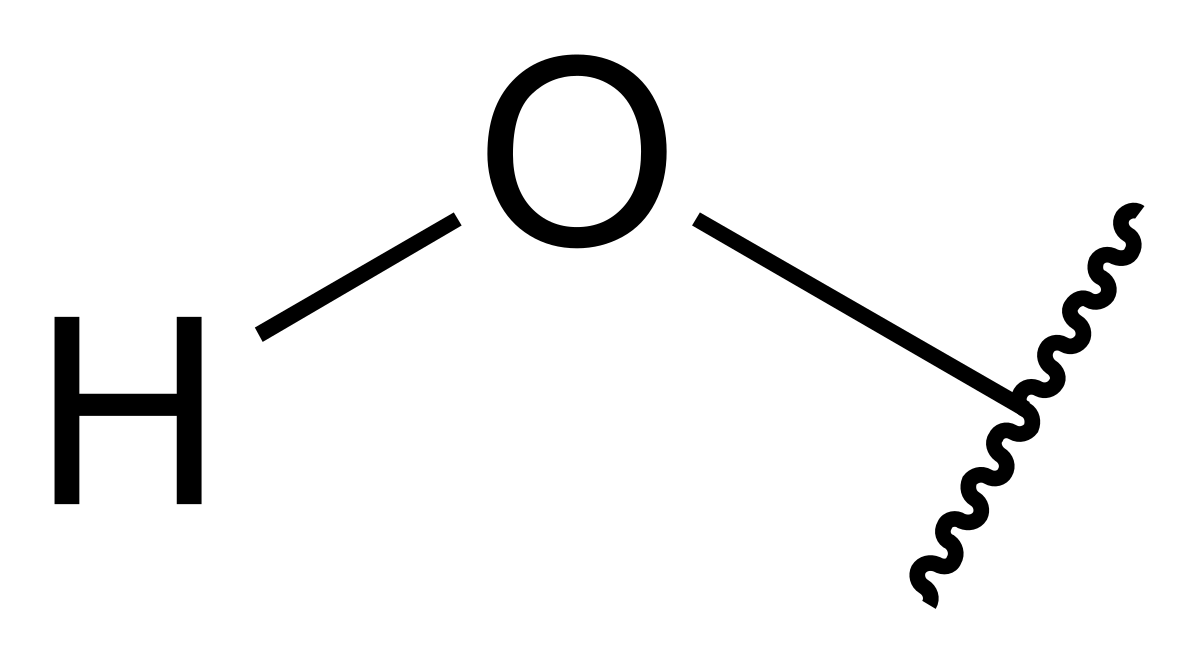
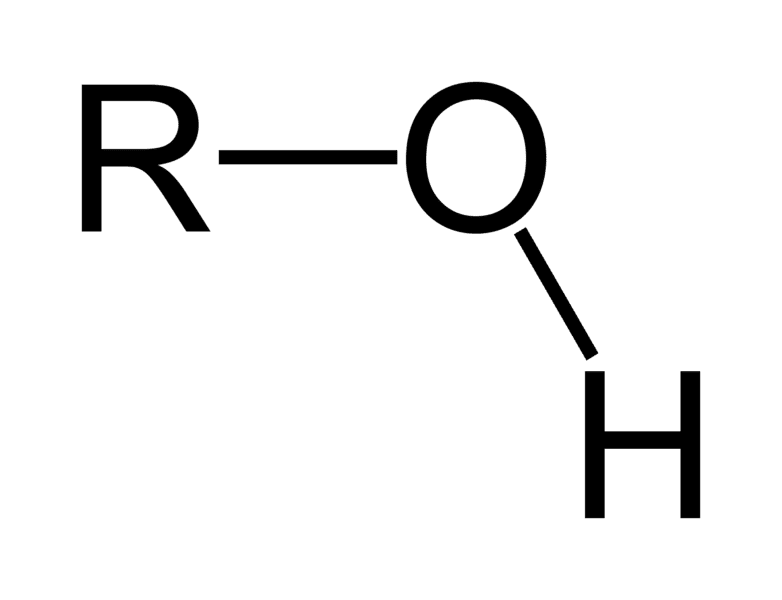
Hydroxyl group
OH
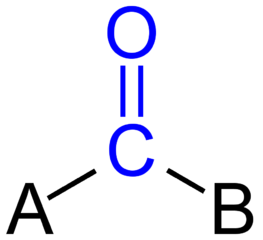
Carbonyl group
CO
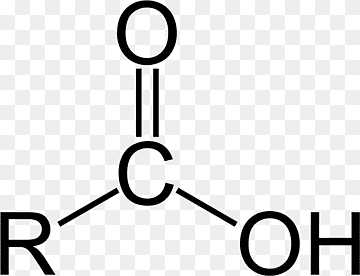
Carboxyl group
COOH
Amino group
NH2
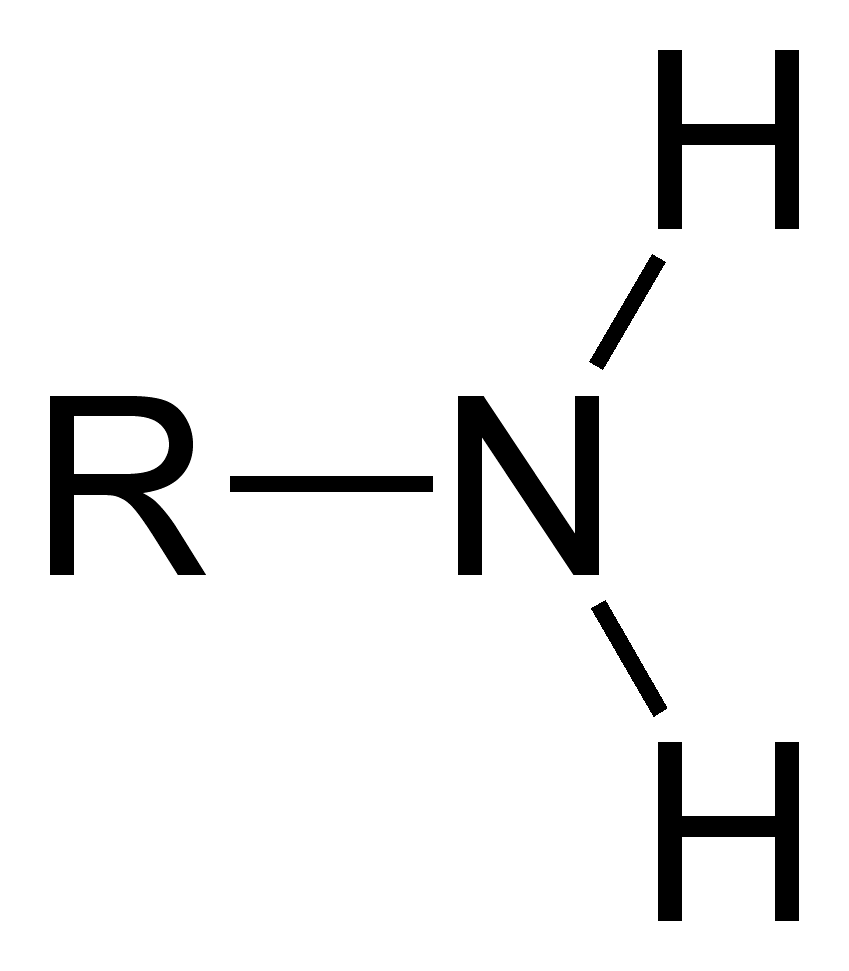
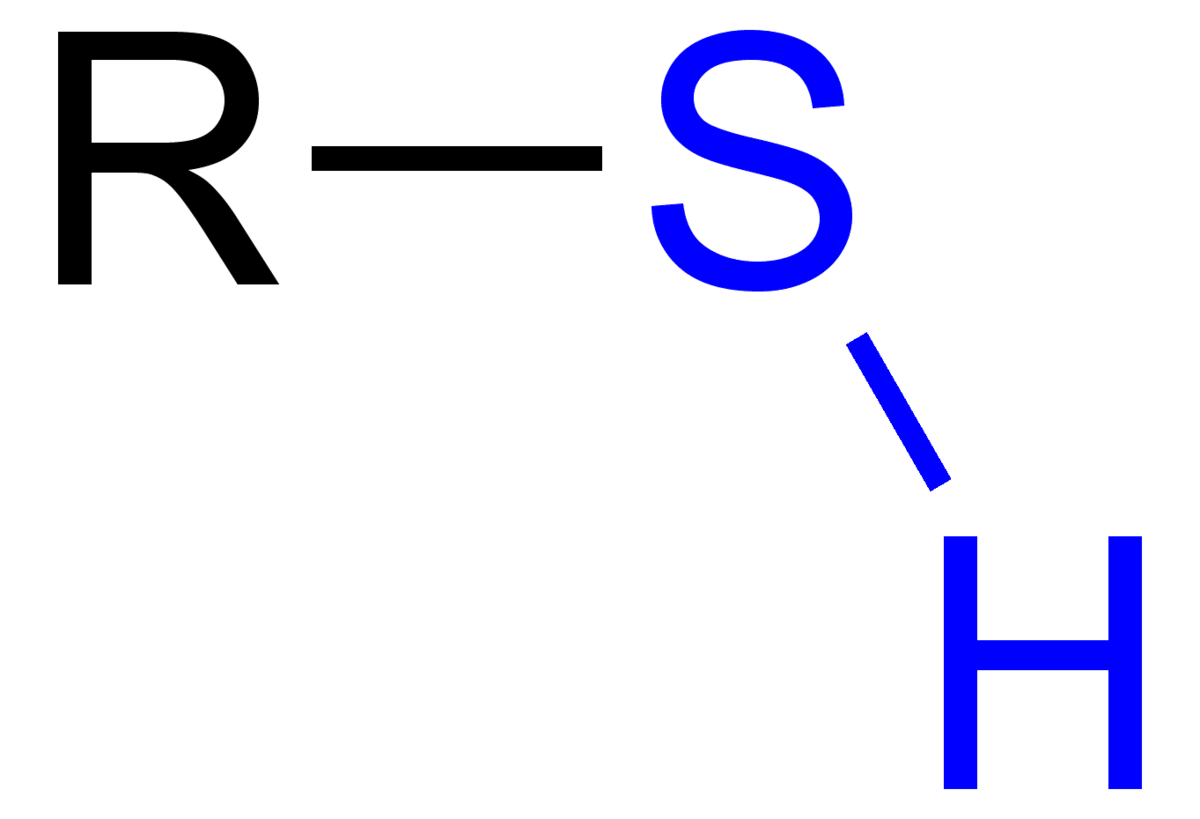
Sulfhydryl group
SH
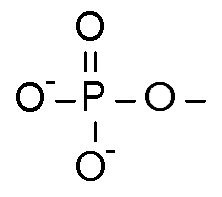
Phosphate group
PO4
groups + structure of Amino Acids
Amino group, carboxyl group, R group (varies), central Carbon, a Hydrogen
Groups + structure of Nucleic Acids
Phosphate group, Nitrogenous base, pentose sugar (C,H,O)
Groups + structure of lipids
Glycerol , carboxyl group, Hydrocarbon Chain
What is the general ratio of a carbohydrate?
CH2O
Metabolism/Metabolic Processes
organisms are characterized by this - chemical reactions that either break down molecules or build them
Intramolecular Bonds
bonds between atoms to make a molecule
Orbitals
volumes of space around the nucleus where electrons are found
Valence Electrons
electrons located in the outermost orbitals that determine an atom's chemical behaviour. if the outermost layer is full, the atom is stable and won't react. if it is not full, it will attempt to gain, lose, or share electrons with other atoms to have full orbitals.
Interactions Between Valence Electrons
they are the root cause of chemical reactions and are responsible for the formation of chemical bonds in atoms.
Two Common Bonds
ionic bonds and covalent bonds
Ionic Bond
a force of attraction between cations and anions. when atoms lose electrons, they become positively charged ions called cations (metal). when atoms gain electrons, they become negatively charged ions called anions (non-metal).
Covalent Bond
is formed when two atoms share one or more pairs of valence electrons. occurs between two non-metals.
Electronegativity
a measure of an atom's ability to attract a shared electron pair when it's participating in a covalent bond.
Nonpolar Covalent Bond
if the atoms attract equally (same electronegativity number) then they will form this bond
Polar Covalent Bond
if one atom attracts electrons more than the other, they will form this bond.
Are covalent or ionic bonds stronger?
covalent
Molecular Polarity
depends on bond polarity and molecular shape. symmetrical molecular structures are nonpolar molecules (regardless of bond polarity). asymmetrical molecular structures are nonpolar if bonds are nonpolar and polar if even one bond is polar.
VSEPR Stands For
valence shell electron pair repulsion
Valence Shell Electron Pair Repulsion
predicts the shape of a molecule. valence electron pairs repel one another and will move as far apart a possible. nonbonding pairs of electrons occupy more space than bonding pairs.
Intermolecular Bonds
bonds between molecules. they are weaker than intramolecular bonds.
Three Types of Intermolecular Bonds
london forces, dipole-dipole forces, and hydrogen bonds
London Forces
weakest bond. formed by unequal distribution of electrons as they move around the nucleus. the unequal electron clouds attract the nucleus of neighbouring atoms. they form between noble gases and nonpolar molecules.
Dipole-Dipole Forces
the partially positive side of one polar molecule attracts the partially negative side of an adjacent polar molecule.
Hydrogen Bonds
the strongest bond. form between an electropositive H of one polar molecule and an electronegative N, O, or F of a neighbouring polar molecule.
Chemicals in our Bodies
with the exception of water, virtually all are carbon-based
Carbon Atoms
a small and relatively light element that has four single valence electrons. carbon atoms attach to each other to form straight and branched chains and ring structures of various sizes and complexity that act as the backbones of biological molecules.
Functional Groups
reactive clusters of atoms. formed from hydrogen, oxygen, sulphur, nitrogen, and/or phosphorus attaching to a carbon backbone.
Hydroxyl Functional Group
Carbonyl Functional Group
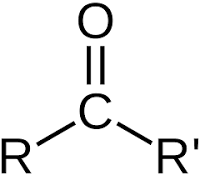
Carboxyl Functional Group
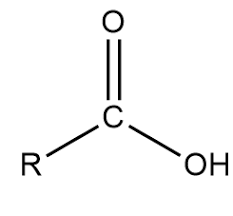
Amino Functional Group
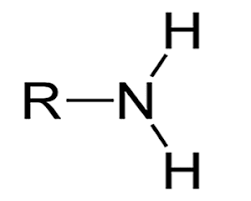
Sulfhydryl Functional Group
Phosphate Functional Group
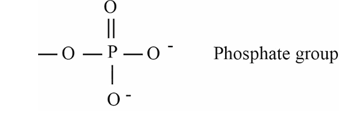
Biological Macromolecules
large molecules sometimes composed of a large number of repeating subunits
Two Reactions Used to Put Subunits Together and Break Them Down
dehydration synthesis (condensation reaction) and hydrolysis
Dehydration Synthesis (Condensation Reaction)
a reaction that creates a covalent bond between two interacting subunits, linking them together. removal of an H⁺ and an OH⁻ to make H₂O. leaves two spots for molecules to join.
Hydrolysis
a reaction in which a water molecule is used to break a covalent bond, holding subunits together.
Four Major Classes of Macromolecules
carbohydrates, lipids, proteins, and nucleic acids
Carbohydrates
nutrients made up of a single sugar molecule or many sugar molecules. they are the most common organic material on earth. plants produce it through photosynthesis (we can't produce it). they are the primary energy source for cells, the structural material of plant cell walls, and cell surface markers for cell-to-cell identification and communication. they are the largest component of our diets. they can be quickly utilized and are easy to break down. it contains carbon, hydrogen, and oxygen in a 1:2:1 ratio.
Three Types of Carbohydrates
monosaccharides, disaccharides, polysaccharides
Monosaccharide
contains a single chain of carbon atoms to which hydroxyl groups are attached. sugar made up of a single sugar molecule (ex. glucose, fructose). they are distinguished from one another by the carbonyl group they possess, the length of their carbon chains, and the spatial arrangement of their atoms (isomers).
Isomer
molecules with the same chemical formula but with different arrangement of atoms. different shapes and physical and chemical properties.
Disaccharide
a sugar made up of two sugar molecules. formed through dehydration synthesis.
Polysaccharide
monosaccharide polymers composed of several hundred to several thousand monosaccharide subunits held together by glycosidic linkages. formed of many sugar molecules linked together (ex. starch, cellulose, and glycogen).
Starch
storage polysaccharide. enzymes in the chloroplasts link excess glucose molecules to one another to form starch, the main energy storage molecule in plants. plants store it as insoluble granules within chloroplasts, amyloplasts, and other plastids.
Glycogen
storage polysaccharide. excess glucose molecules are linked together to form this, which is an energy storage molecule in animals. humans and animals store small amounts in muscle and liver cells and they are converted to glucose/energy during exercise. stores are small and deplete in one day in not replenished.
Cellulose
structural polysaccharide. primary structural polysaccharide of plants and major component of plant cell walls. most abundant organic substance on earth.
Lipids
hydrophobic molecules composed of carbon, hydrogen, and oxygen
Four Families of Lipids
fats, phospholipids, sterols (steroids), waxes
Fats
most common energy storing molecule. one gram of fat stores more than twice the chemical energy of one gram of carbohydrate or protein. animals convert excess carbohydrates into fat and store it as droplets in the cells of fat tissues. the most common fats in plants and animals are triglycerides.
Forming a Triglyceride
dehydration synthesis takes place between a hydroxyl group of glycerol and the carboxyl groups of fatty acids. the resulting bond is called an ester linkage.
Types of Fatty Acids
saturated and unsaturated
Saturated Fatty Acids
have only single bonds between carbon atoms. contain the maximum number of hydrogen atoms possible. solid at room temperature because their straight chains fit closely together (ex. butter and lard).
Unsaturated Fatty Acids
have one or more double-bonded carbon atoms. the double bonds are formed by removing hydrogen atoms. they have less than the max number of hydrogen atoms. liquid at room temperature because the carbon chain is bent, preventing strong intermolecular bonds (ex. olive oil, vegetable oil).
Phospholipids
make up cell membranes, composed of a glycerol molecule attached to two fatty acids and a highly polar phosphate group. water and other polar molecules cannot pass through the bi-layer because of a highly non-polar centre. they must go through proteins in the membrane.
Sterols (Steroids)
used to send signals throughout the body. they are compact hydrophobic molecules containing four fused hydrocarbon rings and several different functional groups (ex. cholesterol - important part of cell membranes, bile, sex hormones - estrogen and testosterone)
Wax
forms a waterproof coating on various plants and animals (ex. apples, bird feathers, and honeycombs). lipids containing long chain fatty acids linked to alcohols or carbon rings.
Proteins
the genetic information in DNA codes specifically for the production of proteins, nothing else. they accomplish more tasks than any other group of biological molecules. proteins are made up of amino acids.
Amino Acids
there are twenty different ones. eight are considered essential, meaning our bodies can't make them from simpler compounds so they must be obtained from our diet. all amino acids have a carboxyl group, an amino group, and a hydrogen bonded to the central carbon. two or more are bonded together through a condensation reaction. the carboxylic acid group of one bonds to the amino group of another through the removal of water. billions of proteins can be made with this. if proteins are words, amino acids are letters.
Structures of Proteins
primary, secondary, tertiary, and quaternary
Primary Structure of Protein
the sequence of amino acids in the polypeptide strand. protein is made up of a polypeptide chain of amino acids and peptide bonds. the order of amino acids determines it.
Secondary Structure of Protein
hydrogen bonds that form with nearby amino acids coil and fold the polypeptide into α helices and β-pleated sheets. hydrogen bonds between the carboxyl groups and amino groups hold it in these two shapes.
Tertiary Structure of Protein
the polypeptide folds further into this structure. these folds are stabilized by R group-R group interactions. the structure holds in place because hydrophobic ends, hydrogen and ionic bonds, van der waal forces, proline kink (functional group with an amino group), and disulphide bridge (cystine contains sulphur and when brought close to another sulphur, it forms a covalent bond).
Quaternary Structure of Protein
the clustering of two or more polypeptides in tertiary structure. shape matters significantly, it determines how the protein will behave. several polypeptides in a tertiary structure interact and the functional groups react.
Denaturing of Proteins
refers to a change in the 3D shape of a protein. it can no longer carry out its biological function, which can be dangerous or helpful.
What can denature proteins?
temperature, pH, and salt concentration
Temperature Denaturing Proteins
temperature can cause hydrogen bonds to destabilize. a prolonged fever above 39°C can denature enzymes in the brain leading to seizures and possibly death. heat can denature proteins in hair so we can straighten or curl it temporarily.
pH Denaturing Proteins
pH destabilizes hydrogen and disulphide bonds. pepsin is a protein-digesting enzyme in the stomach that works best at a pH of 2 (pH in the stomach) and is denatured in the small intestine where the pH is increased to approximately 10.
Salt Concentration Denaturing Proteins
affects hydrophobic proteins. curing meats with high concentrations of salt or sugar preserves food by denaturing the enzymes in the bacteria that would otherwise spoil the food.
What do organisms need to do before they can use energy?
in most cases, organisms obtain energy in one form and convert it into another before it can be used. the transfer of energy follows the laws of thermodynamics. (ex. plants convert light energy to chemical potential energy through photosynthesis. glucose becomes atp through cellular respiration)
The First Law of Thermodynamics
the total amount of energy in the universe is constant. energy cannot be created or destroyed but only converted from one form to another. if an object or process gains an amount of energy, it does so at the expense of a loss of energy somewhere else in the universe. all reactions can be identified as either endergonic or exergonic.
Endergonic Reaction
a chemical reaction in which the energy of the products is more than the energy of the reactants. this reaction takes in energy. (ex. photosynthesis)
Exergonic Reaction
a chemical reaction in which the energy of the products is less than the energy of the reactants. the reaction releases energy. (ex. gasoline)
The Second Law of Thermodynamics
the entropy of the universe increases with any change that occurs.
Entropy
a measure of the randomness or disorder in a collection of objects or energy.
Do living organisms violate the second law of thermodynamics?
they seem to but don't. for example, plants make a very ordered glucose molecule out of disordered light. the apparent order created by photosynthesis is accompanied by an even greater disorder of the sun as it converts hydrogen to helium and releases a host of subatomic particles including light that is used by the plant. photosynthesis creates order at the expense of a greater disorder of the sun.
Enzyme
a protein molecule that acts as a catalyst
Catalyst
a substance that speeds up a chemical reaction without being consumed in the process.
Activation Energy in Chemical Reactions
all chemical reactions require activation energy. heat provides the activation energy for many reactions (ex. spark plug in an engine). however, living cells cannot survive high levels of heat so they use enzymes. many enzymes decrease the activation energy by stretching and bending chemical bonds. some enzymes require cofactors or coenzymes before they can work properly.
Substrate
the reactant that an enzyme acts on when it catalyzes a chemical reaction.
Structure of Enzymes
they are proteins in tertiary or quaternary structure with complex formations. they are very specific for the substance to which they bond.
Model of Enzyme Activity
the active site is ready to receive the substrate. the active site is usually a pocket or groove in the 3D structure of the enzyme.
the substrate enters the active site and forms weak bonds.
substrate is converted to products.
products are released and the enzyme is available for another reaction.
Cofactors
inorganic substances such as zinc and manganese ions.
Coenzymes
organic substances that are often derivatives of many vitamins.
Ways Enzyme Activity is Regulated
enzyme inhibition, allosteric regulation, and feedback inhibition
Enzyme Inhibition
a variety of substances inhibit enzyme activity. there are competitive inhibitors and noncompetitive inhibitors.
Competitive Inhibitor
so similar to the enzyme's substrate that they are able to enter the enzyme's active site and block the normal substrate from binding. the process is reversible and can be overcome by increasing the substrate concentration.
Noncompetitive Inhibitor
they attach to another site on the enzyme (not the active site), causing a change in the enzyme's shape. this changes the active site in such a way that it loses affinity for its substrate. (ex. DDT inhibits enzymes of the nervous system)
Allosteric Regulation
uses the allosteric sites, activators, and allosteric inhibitors. noncompetitive inhibitors attach to the allosteric sites of certain enzymes. the binding of an activator or inhibitor to one of the allosteric sites will affect the activity of all the active sites on the enzyme. allosteric regulations attach to their sites using weak bonds.