Alcohols
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What functional group do alcohols contain
Hydroxyl (-OH) functional group.
Name and draw first 8 alcohols
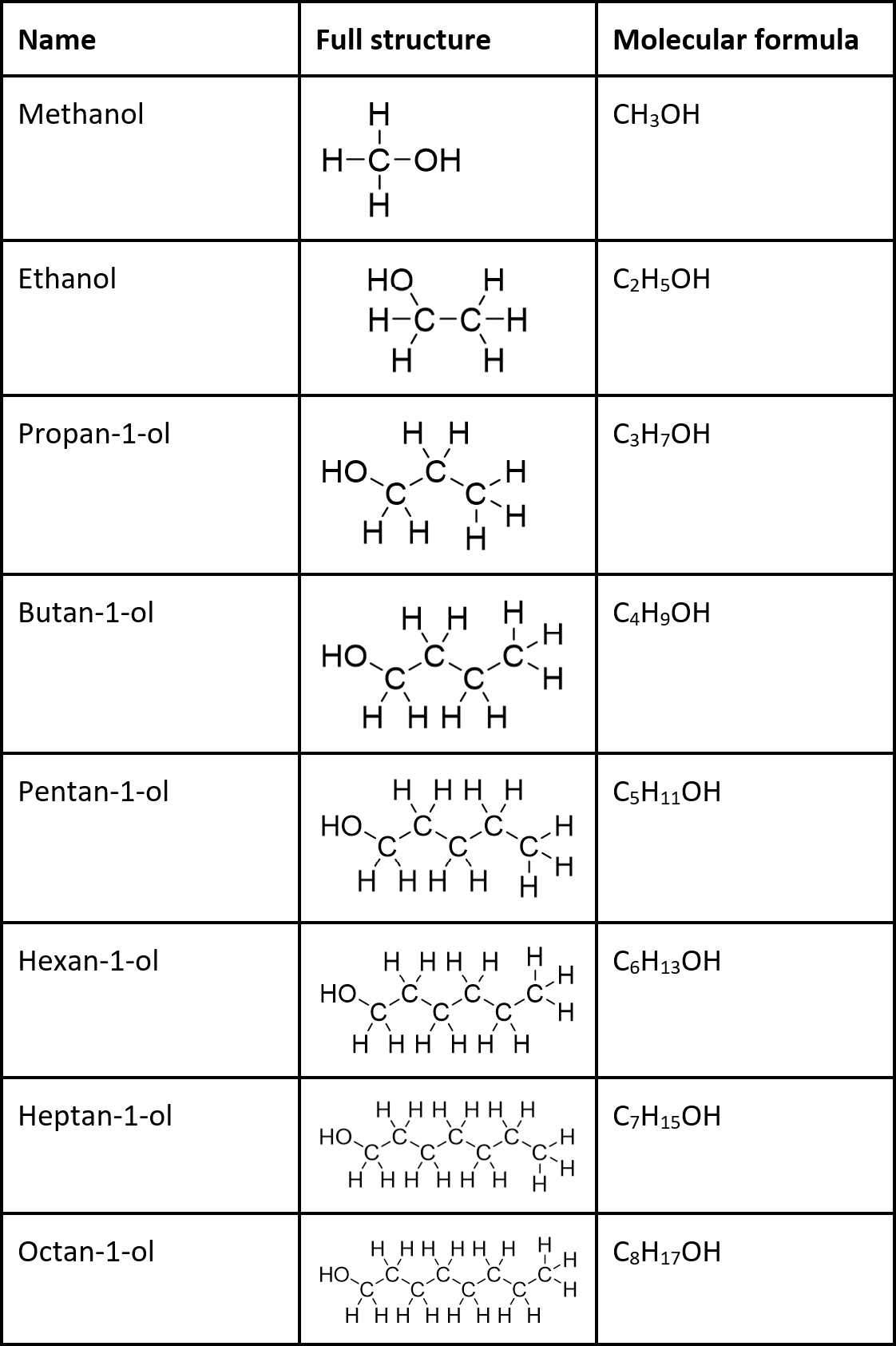
When naming alcohols, the name ends in -ol and you must state the position of the -OH group in the molecule. Remember to number it on the lowest carbon possible.
How can alcohols be classed
-Primary
-Secondary
-Tertiary
Describe a primary alcohol
OH group attached to a C atom which has 2 other H’s (Or C atom the OH is connected to is only connected to 1 other C atom). This only occurs when the -OH group is at the end of the molecule.
-Propanol
Describe a secondary alcohol
-OH is attached to a carbon with only one hydrogen atom attached (or C atom OH is attached to has 2 other C atoms).
This can happen somewhere in the middle of a carbon chain.
-Propan-2-ol
Describe a tertiary alcohol
-OH group is attached to a C atom with no other hydrogen atoms attached ( or C atom attached to 3 other C atoms).
This will mean that the hydroxyl group is joined to the same C atom as a branch
-2-methylpropan-2-ol
What is a diol
An alcohol with 2 hydroxyl groups
What is a triol
An alcohol with 3 hydroxyl groups
Explain the effect the hydroxyl group has on an alcohol’s physical properties
Alcohols with more hydroxyl groups will have higher melting and boiling points (due to greater degree of hydrogen bonding)