Biochem 1 FInal exam study
1/389
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
390 Terms
What is the mnemonic for the enzymes of the glycolytic pathway?
Hungry Pirates Pick All the Greatest Pickled Pumpkins Ever Picked
(Hexokinase, Phosphoglucose isomerase, Phosphofructokinase, Aldolase, Triose Phosphate Isomerase, Glyceraldehyde 3-phosphate dehydrogenase, Phosphoglycerate Kinase, Phosphoglycerate Mutase, Enolase, Pyruvate Kinase)
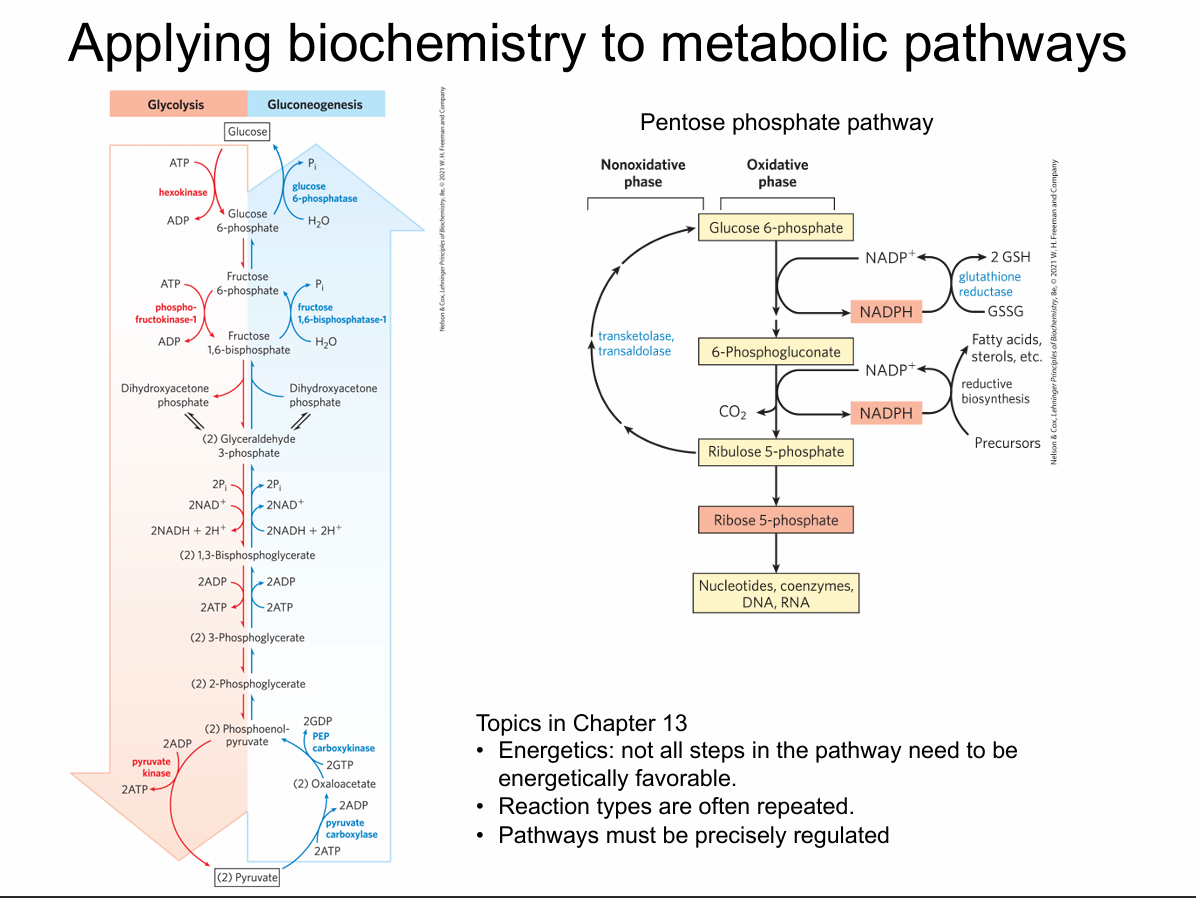
What does “applying biochemistry to metabolic pathways” mean in the context of this slide?
It means using biochemical principles like thermodynamics, reaction types, and regulation to understand how multi-step metabolic pathways (such as glycolysis, gluconeogenesis, and the pentose phosphate pathway) function as coordinated sequences of enzyme-catalyzed reactions.
What is the pentose phosphate pathway (PPP) in broad terms?
The PPP is a metabolic pathway that branches from glucose-6-phosphate and primarily generates NADPH (for reductive biosynthesis and antioxidant defense) and ribose-5-phosphate (for nucleotide synthesis), with both oxidative and nonoxidative phases.
What are the two major phases of the pentose phosphate pathway shown in the diagram?
Oxidative phase – converts glucose-6-phosphate into ribulose-5-phosphate, producing NADPH and CO₂.
Nonoxidative phase – rearranges sugar phosphates (C3–C7) to regenerate glycolytic intermediates or ribose-5-phosphate as needed.
Why is it useful that some steps in a pathway (like PPP) are near equilibrium while others are highly exergonic?
Near-equilibrium steps easily reverse and help the pathway adapt to changing substrate/product levels.
Highly exergonic steps act as committed, regulated points that drive the pathway forward and often serve as key control nodes.
A cell suddenly needs more NADPH for fatty acid synthesis. How could regulation of the PPP help meet this demand?
The cell can upregulate the oxidative phase (e.g., activating glucose-6-phosphate dehydrogenase) to increase NADPH production and adjust flux through the nonoxidative phase to balance sugar phosphates with glycolysis.
how are actual free energy changes related to standard free energy changes?
Actual ΔG depends on ΔG′° and the actual concentrations of reactants and products via the relationship
ΔG = ΔG′° + RT ln Q,
where Q is the mass-action ratio.
Who were Antoine Lavoisier and Marie Anne Pierette Paulze in the context of metabolism?
Lavoisier was an 18th-century chemist often called the “father of modern chemistry,” and Paulze was his collaborator and scientific partner; together, they performed early experiments that linked respiration to combustion.

What did Lavoisier’s calorimeter experiment show about respiration?
It showed that a respiring guinea pig produced heat and CO₂ in a way comparable to a burning candle, demonstrating that respiration is a controlled combustion process in living organisms.
What famous quote from Lavoisier describes respiration?
“Respiration is nothing but a slow combustion of carbon and hydrogen, which is entirely similar to that which occurs in a candle.”
What is a calorimeter
A device that measures heat released during a process—in this case, used to quantify heat from respiration and compare it to heat from combustion.
How does Lavoisier’s view of respiration connect to modern bioenergetics?
Modern bioenergetics still views cellular respiration as stepwise oxidation of carbon-based fuels, where energy from electron transfer is captured in ATP instead of being released all at once as heat.
Why is it advantageous for cells that respiration is “slow combustion” rather than a literal flame?
Slow, stepwise combustion allows cells to capture free energy in usable forms (ATP, ion gradients) rather than losing it as uncontrolled heat that would damage cellular structures.
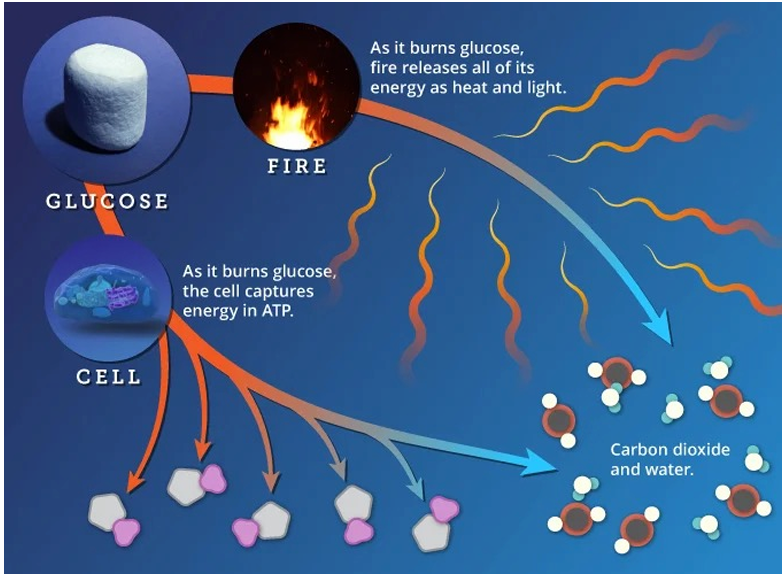
What key statement about free-energy change is made on this slide?
The free-energy change (ΔG) for a reaction is independent of the pathway by which the reaction occurs.
What does it mean that ΔG is pathway-independent?
Whether glucose is oxidized by direct combustion (fire) or by many enzyme-catalyzed steps in a cell, the overall ΔG for converting glucose + O₂ → CO₂ + H₂O is the same; only the rate and control differ.
What role do enzymes play if they do not change ΔG?
Enzymes lower the activation energy (ΔG‡) and increase the rate of the reaction, but they do not alter equilibrium concentrations or the net free-energy change.
If enzymes do not change ΔG, how can they still allow otherwise “impossible” reactions to occur in cells?
Many reactions have a favorable ΔG but prohibitively high activation energy; enzymes lower this barrier so that reactions happen at biologically useful speeds without changing thermodynamic favorability.
In the context of glucose oxidation, why is the stepwise enzymatic pathway preferred over a single combustion step?
The stepwise pathway allows controlled capture of free energy in ATP and reducing equivalents and avoids cell damage that would occur if all energy were released explosively as heat and light.
What is the First Law of Thermodynamics?
It is the principle of conservation of energy: energy cannot be created or destroyed, only transformed from one form to another.
What is “energy transduction”?
The process of converting one form of energy into another, such as converting light energy to chemical energy or chemical energy to mechanical work or heat.
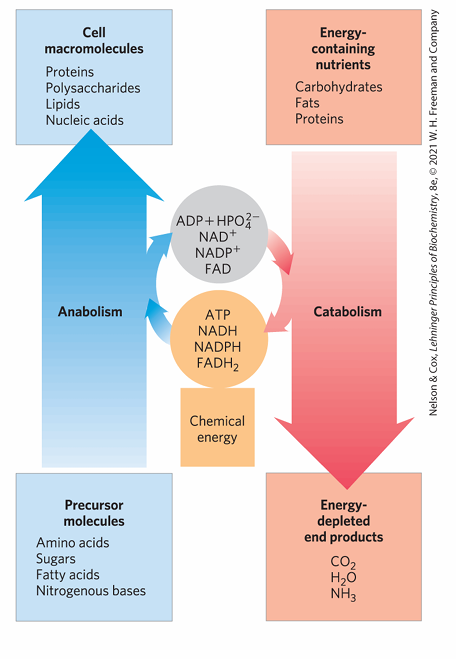
According to the slide’s metabolic diagram, what is the role of catabolism and anabolism in energy transduction?
Catabolism breaks down energy-containing nutrients (carbohydrates, fats, proteins) to smaller, energy-depleted products, producing ATP, NADH, and other energy carriers.
Anabolism uses ATP, NADH, etc., to build cell macromolecules (proteins, polysaccharides, lipids, nucleic acids) from precursor molecules.
A plant converts sunlight into stored energy in glucose. Which law of thermodynamics explains this, and how?
The First Law: the plant does not create energy, but transduces light energy into chemical bond energy stored in or organic molecules.
How does the First Law apply when a person eats food and then generates heat while running?
Chemical energy from food is transduced into ATP, mechanical work (muscle contraction), and heat; the total energy is conserved, but its form changes.
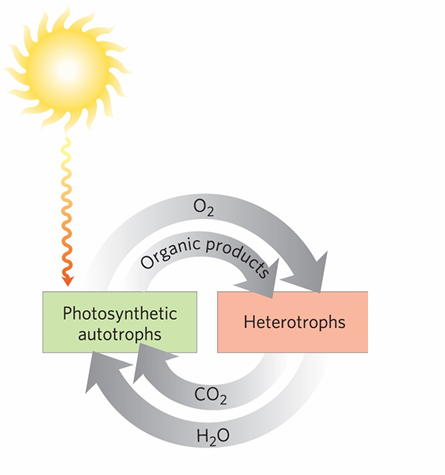
In the circular diagram showing photosynthetic autotrophs and heterotrophs, what is the overall energy flow?
Energy enters the biosphere as sunlight captured by photosynthetic autotrophs, becomes chemical energy in organic products, is used by heterotrophs and plants via catabolism, and is ultimately released back as heat to the surroundings.
What does the Second Law of Thermodynamics state?
The universe always tends toward increasing entropy (greater multiplicity or disorder).
What is a “reacting system” ?
The specific collection of matter undergoing a particular chemical or physical process, which can be considered closed or open.
What is the “universe” in thermodynamic terms?
The reacting system plus its surroundings.
Are living cells closed or open systems, and what does that mean?
Living cells are open systems: they exchange matter and energy with their surroundings (e.g., nutrients in, heat and waste out).
How can a cell maintain or even increase its internal order without violating the Second Law?
By importing low-entropy nutrients (order) and exporting higher-entropy waste and heat, such that the overall entropy of the universe increases even while cellular order increases.
What would be an example of a closed biological system, and why is it mostly theoretical?
A perfectly insulated, sealed cell with no exchange of matter or energy would be a closed system—but real cells cannot survive like this, so in practice biological systems are never perfectly closed.

How do living organisms “take” and “return” free energy according to this slide?
They take free energy from the surroundings as sunlight or nutrients and return an equal amount (or more) as heat and entropy, maintaining compliance with the Second Law.

How does this slide conceptually connect metabolism with evolution of complexity?
It shows that increasing biological complexity and organization (e.g., multicellular organisms) can arise as long as it is energetically powered and accompanied by even greater entropy production elsewhere.
What is the Gibbs free energy equation ?
ΔG = ΔH – TΔS.
Define G, H, T, and S in the Gibbs Free Energy Equation.
G – Gibbs free energy (usable energy available to do work).
H – Enthalpy (heat content related to chemical bonds).
T – Absolute temperature (Kelvin).
S – Entropy (multiplicity or disorder).
Under what conditions is ΔG used in biochemistry?
At constant temperature and pressure, which is a good approximation for cellular conditions.
If a reaction has ΔH = -40 kJ/mol and ΔS = -0.05 kJ/mol·K at 298 K, calculate ΔG and state if it is favorable.
ΔG = ΔH − TΔS
= (-40) − 298(−0.05)
= -40 + 14.9 ≈ -25.1 kJ/mol.
Negative ΔG → exergonic, reaction is favorable in the forward direction.
For a reaction with ΔH = +10 kJ/mol and ΔS = +0.07 kJ/mol·K at 298 K, calculate ΔG. Is it enthalpy- or entropy-driven?
ΔG = 10 − 298(0.07)
= 10 − 20.86 ≈ -10.9 kJ/mol (favorable).
The reaction is entropy-driven because enthalpy is unfavorable but the positive ΔS term dominates.
How can a reaction with positive ΔH still have a negative ΔG?
If ΔS is sufficiently positive and the temperature is high enough so that the TΔS term outweighs ΔH, making ΔG negative and the reaction thermodynamically favorable.
What does a negative ΔG mean for a reaction?
The reaction is exergonic, releases free energy, and tends to proceed spontaneously in the forward direction under the given conditions.
What does a positive ΔG indicate?
The reaction is endergonic, requires input of free energy, and will proceed in reverse (toward reactants) unless coupled to a favorable process.
What does ΔG = 0 mean?
The reaction is at equilibrium; forward and reverse rates are equal and there is no net change in concentrations or free energy.
If ΔG for a metabolic step is slightly positive in standard conditions, how might a cell still run that step forward?
By adjusting concentrations (high substrate, low product), by coupling the step to a strongly exergonic reaction (e.g., ATP hydrolysis), or embedding it in a pathway where the overall ΔG is negative.
Why is it important that most metabolic pathways operate far from equilibrium at their regulated steps?
Steps with large negative ΔG are irreversible in vivo and therefore good control points for regulating flux; near-equilibrium steps cannot strongly control flux because they can easily reverse.
How is enthalpy (H) defined ?
As the heat content of the reacting system, reflecting the number and kinds of chemical bonds (covalent and noncovalent) in reactants and products.
What happens to heat when a chemical bond is created vs broken?
Creating a bond releases heat.
Breaking a bond requires heat.
What does a negative ΔH indicate about a reaction?
The reaction is exothermic, releasing heat to the surroundings.
What does a positive ΔH indicate?
The reaction is endothermic, absorbing heat from the surroundings.
A reaction has ΔH < 0 and ΔS < 0. Under what conditions (high vs low temperature) will it be spontaneous?
ΔG = ΔH − TΔS. If both ΔH and ΔS are negative, spontaneity is favored at lower temperatures (small TΔS term), because high T makes the unfavorable −TΔS term more positive.
How is entropy (S) defined
Entropy is dependent on multiplicity (W)—the number of ways the state of a system can be realized—via S = R ln W, where R is the gas constant.
What does a negative ΔS mean for a reaction?
The system’s multiplicity decreases (fewer microstates), often described as becoming more ordered, and this is unfavorable for spontaneity.
What does a positive ΔS mean
Multiplicity increases (more microstates), often described as the system becoming more disordered; this is favorable for spontaneity.
Give a biochemical example where entropy increases (positive ΔS) during a reaction.
Hydrolysis of ATP to ADP + Pi increases the number of particles and the freedom of motion of products, increasing multiplicity and contributing positive ΔS.
Why might two small molecules dimerizing (forming one larger complex) often have negative ΔS?
Because two free molecules can occupy more microstates (positions and orientations) than one bound dimer, so binding often reduces multiplicity and thus entropy.
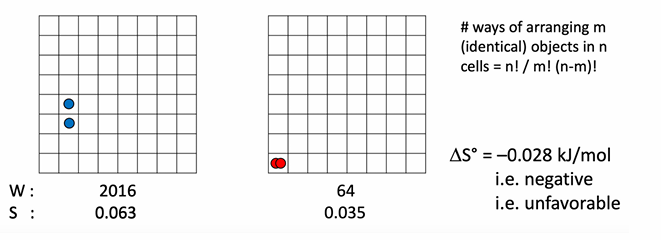
In the entropy diagram with red and blue dots representing particles in boxes, how does the arrangement illustrate a change in multiplicity?
The grid with many possible positions for separate particles has many possible microstates (high W), while grouping them reduces the number of possible arrangements (lower W), visually representing the entropy change as particles associate.
What is the standard transformed free energy change (ΔG′°)?
ΔG′° is the free-energy change of a reaction measured under biochemical standard conditions: pH 7, [H₂O] = 55.5 M, [Mg²⁺] = 1 mM, and all other solutes at 1 M.
How is ΔG′° related to the standard equilibrium constant K′eq?
ΔG′° = −RT ln(K′eq), meaning ΔG′° reflects the position of equilibrium under biochemical conditions.
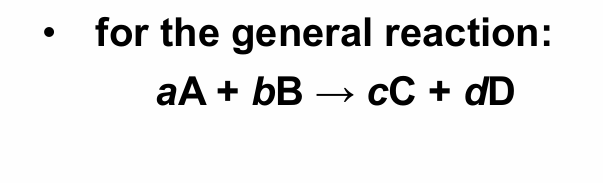
What is the general reaction format represented on this slide?
aA + bB → cC + dD, where lowercase letters are stoichiometric coefficients.
Why do biochemists use K′eq instead of Keq?
Because K′eq accounts for biochemical standard state, especially constant pH (7) and Mg²⁺ binding that affect reactant ionization.
Why is ΔG′° useful for comparing different reactions?
It gives a standardized measure of reaction favorability that is independent of concentration effects.
If two reactions have ΔG′° = −3 and −30 kJ/mol, which is more likely irreversible?
The −30 kJ/mol step because significantly negative ΔG′° indicates an irreversible, regulatory step.
What is the relationship between K′eq and ΔG′° ?
K′eq > 1 → ΔG′° negative → proceeds forward
K′eq = 1 → ΔG′° = 0 → equilibrium
K′eq < 1 → ΔG′° positive → proceeds reverse
Can ΔG′° be experimentally measured?
Yes—by measuring K′eq experimentally, then calculating ΔG′° via ΔG′° = −RT ln K′eq.
Why do regulated metabolic pathways typically have reactions where K′eq >> 1?
Because those steps are strongly exergonic (ΔG′° ≪ 0), preventing backward flux and allowing tight control of pathway flow.
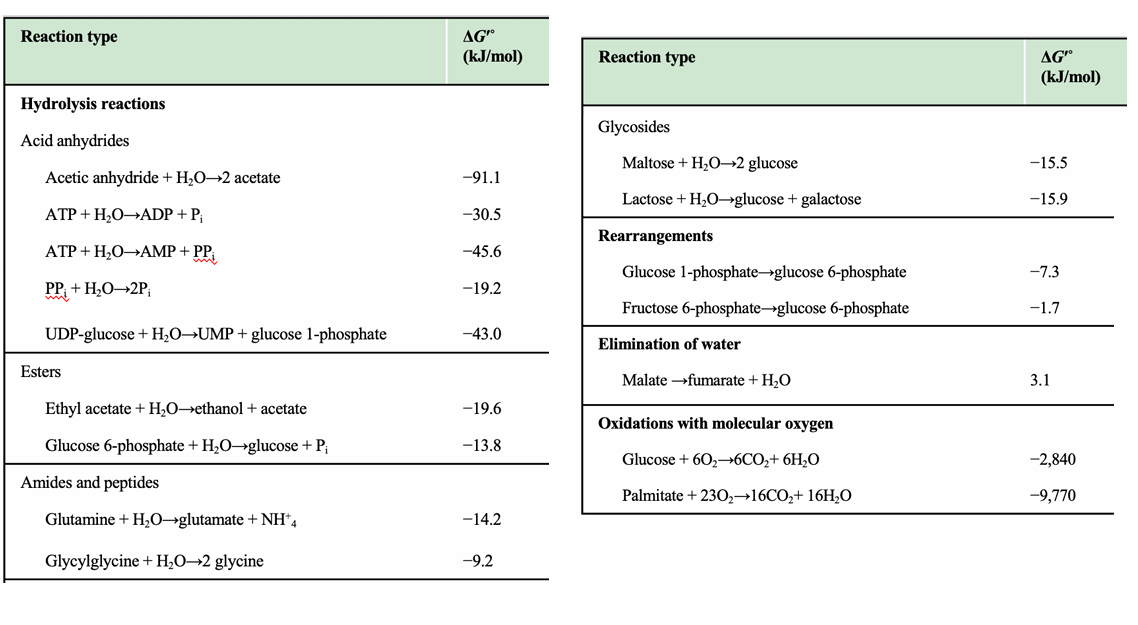
What kind of reactions are listed in the table on this slide?
Hydrolysis reactions, rearrangements, glycosidic bond cleavage, and oxidations.
What trend is visible for anhydride hydrolysis (e.g., ATP → ADP + Pi)?
They have strongly negative ΔG′°, indicating highly favorable hydrolysis.
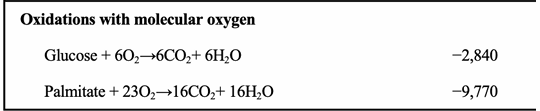
Why do “oxidations with molecular oxygen” have very negative ΔG′° values?
Because they are highly exergonic due to oxygen being a strong electron acceptor.
Why is the negative ΔG′° of ATP hydrolysis important in metabolism?
It allows ATP hydrolysis to drive endergonic reactions via coupling.
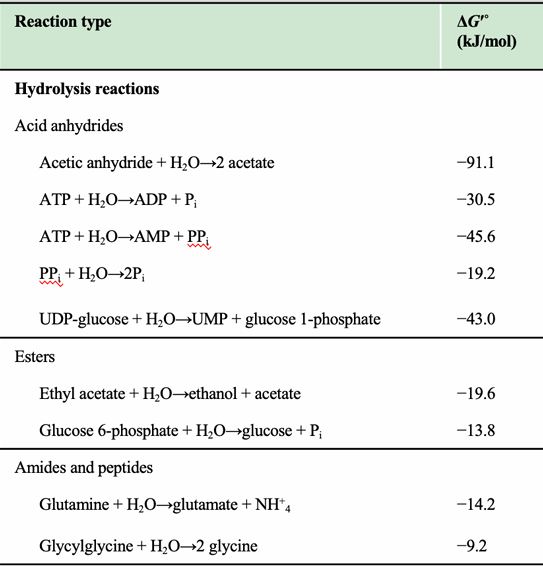
Why is glucose 6-phosphate hydrolysis less exergonic than ATP hydrolysis?
It has fewer destabilizing charges and less resonance gain upon hydrolysis.
What is the mass-action ratio Q?
Q = ([C]^c[D]^d)/([A]^a[B]^b), using actual cellular concentrations.
![<p>Q = ([C]^c[D]^d)/([A]^a[B]^b), using <strong>actual</strong> cellular concentrations.</p>](https://knowt-user-attachments.s3.amazonaws.com/a10b0f03-1e6b-45e4-a514-2116c4831e65.png)
What is the relationship between ΔG and ΔG′°?
ΔG = ΔG′° + RT ln Q.
Why can near-equilibrium reactions reverse easily in cells?
Because ΔG is near zero and small concentration changes shift direction.
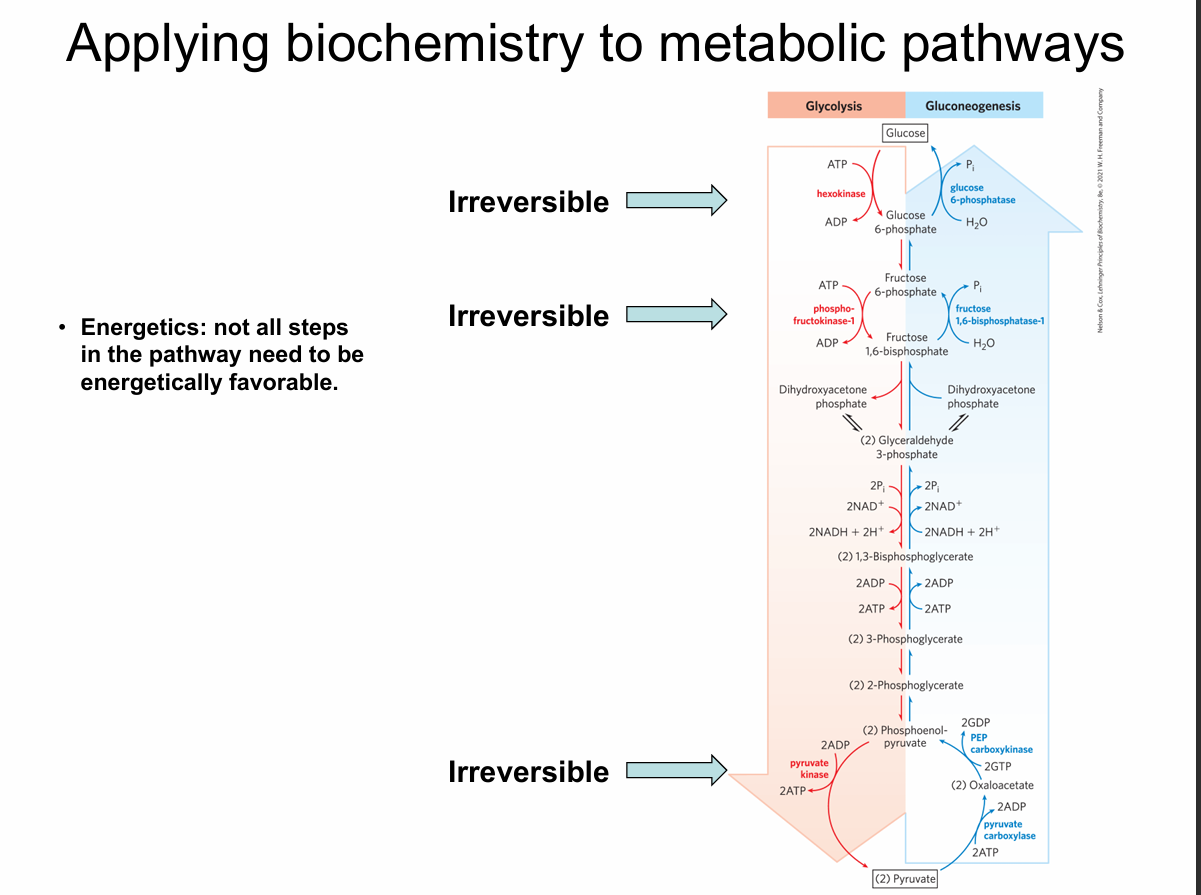
What is emphasized about energetics on this slide?
Not all steps need to be energetically favorable; a few strongly exergonic, irreversible steps drive the pathway.
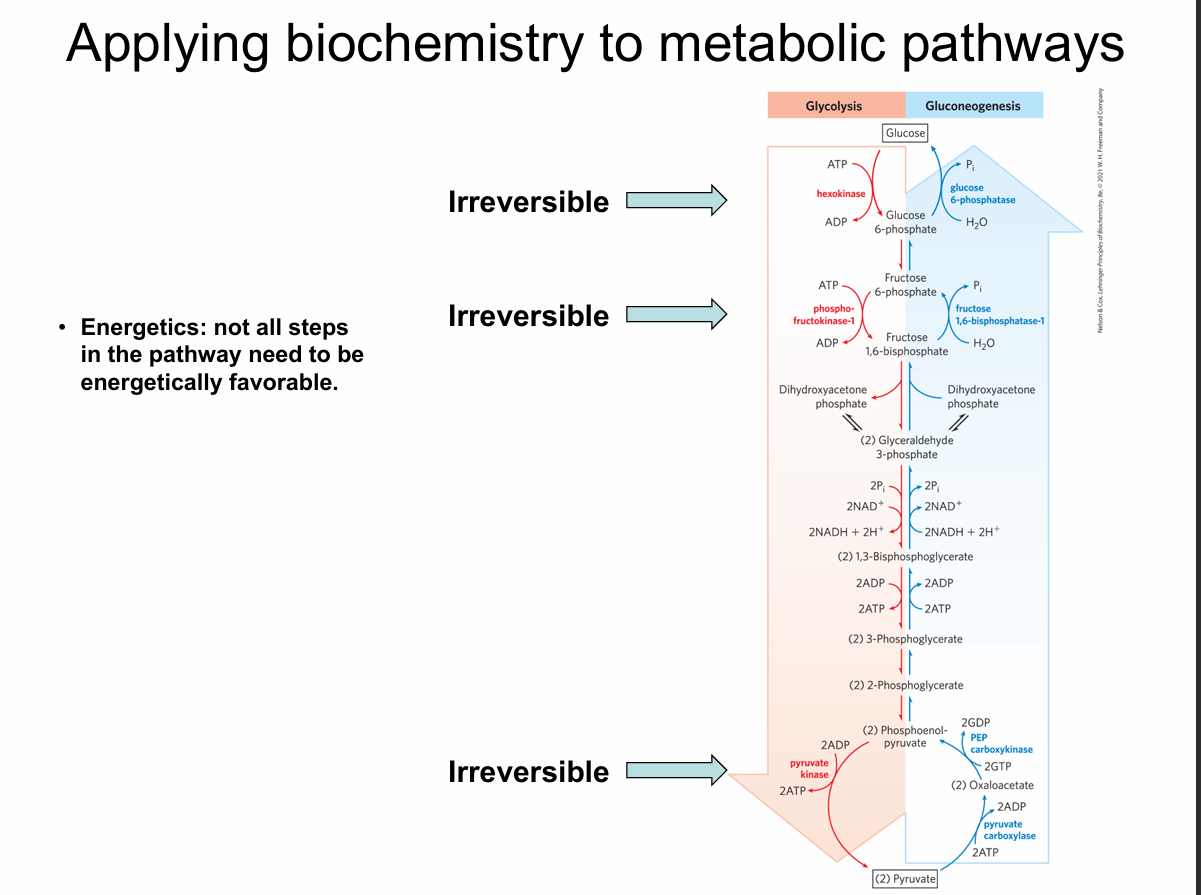
Which steps in glycolysis are shown as irreversible?
Hexokinase, phosphofructokinase-1 (PFK-1), and pyruvate kinase.
Why can’t reversible steps control pathway flux?
Because ΔG ≈ 0, small concentration variations reverse direction; they lack “driving force.”
Why must irreversible steps be regulated?
Why does K′eq < 1 imply ΔG′° is positive?
Because ln(K′eq) is negative → −RT · ln(K′eq) becomes positive.
For K′eq = 10 at 298 K, calculate ΔG′°.
ΔG′∘=−RTln(10)
R = 8.314 J/mol·K
ln(10) = 2.303
ΔG′∘=−(8.314)(298)(2.303)=−5.7 kJ/mol\Delta G'^\circ = -(8.314)(298)(2.303) = -5.7\text{ kJ/mol}ΔG′∘=−(8.314)(298)(2.303)=−5.7 kJ/mol
Why is K′eq tied to reaction energetics?
K′eq reflects the ratio of product to substrate at equilibrium, which encodes the underlying stability differences between reactants and products — the physical basis of ΔG′°.
Why are hydrolysis reactions typically negative ΔG°′?
Hydrolysis converts molecules into more stable, lower-energy products and increases entropy, leading to strongly negative free energy changes.
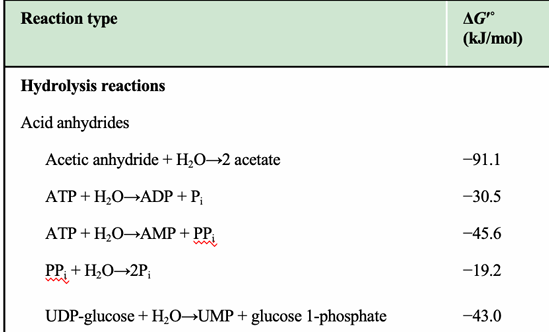
What category do ATP → ADP + Pi and ATP → AMP + PPi belong to?
Acid anhydride hydrolysis reactions, which have large negative ΔG°′ values.
What is PPi hydrolysis classified as?
Hydrolysis of pyrophosphate, giving a ΔG°′ of –19.2 kJ/mol.
Why does ATP → AMP + PPi have a more negative ΔG°′ than ATP → ADP + Pi?
Because hydrolysis of ATP to AMP produces PPi, which is then rapidly hydrolyzed in cells, driving the reaction even further forward.
Why is oxidation of palmitate so exergonic (ΔG°′ = –9,770 kJ/mol)?
Fatty acid oxidation yields massive amounts of CO₂ + H₂O, forming many stable bonds and releasing huge free energy.
Why is malate → fumarate + H₂O positive (ΔG°′ = +3.1 kJ/mol)?
It is an elimination reaction that lowers entropy and creates a less stable intermediate.
Why are rearrangements (e.g., glucose-1-phosphate → glucose-6-phosphate) weakly negative?
They involve minor internal bond shifts without large enthalpy changes.
Why are peptide hydrolysis values only moderately negative?
Breaking amide bonds releases some energy, but peptides are relatively stable, making ΔG°′ modest.
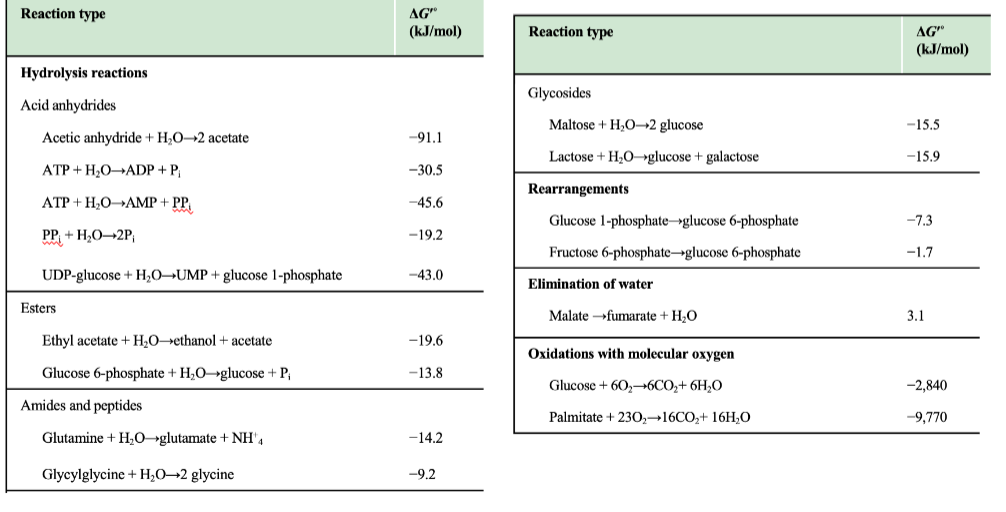
Which class shows the strongest exergonic reactions?
Oxidations with molecular oxygen, far more negative ΔG°′ values than any other category.
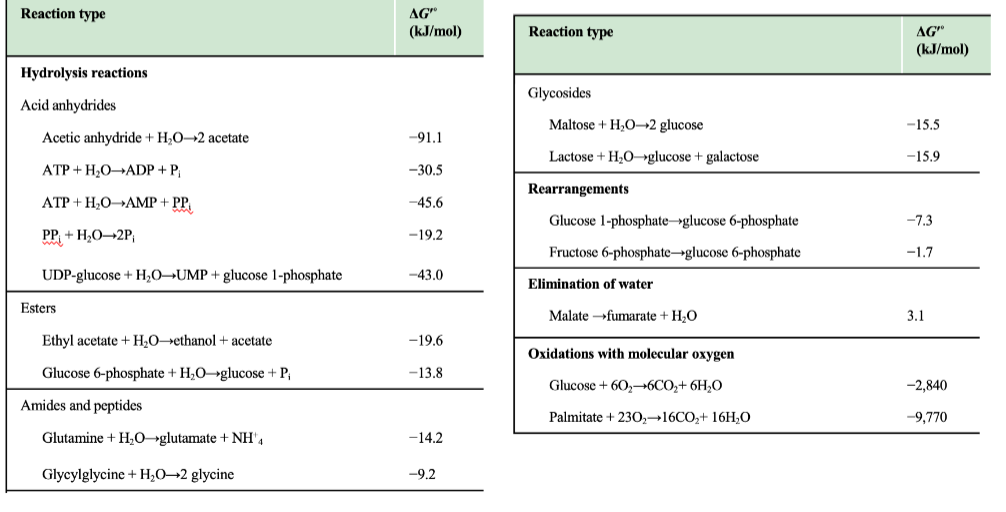
Which ATP reaction is most exergonic?
ATP → AMP + PPi (ΔG°′ = –45.6 kJ/mol), identifiable as the most negative among the ATP entries.
What is the formula linking ΔG and ΔG°′?
ΔG=ΔG′∘+RTln(([A]a[B]b)/([C]c[D]d))
![<p><span>ΔG=ΔG′∘+RTln(([A]a[B]b)/([C]c[D]d))</span></p>](https://knowt-user-attachments.s3.amazonaws.com/938dc9ae-cb34-46a6-ba87-3c955daf131d.png)
What is Q (mass-action ratio)?
The ratio
Q=([C]c[D]d)/([A]a[B])
evaluated at current (not equilibrium) concentrations.
How can a reaction with positive ΔG°′ proceed forward in cells?
If substrate concentrations are high or product concentrations are low, making RT ln Q sufficiently negative.
Why is ΔG more useful than ΔG°′ in biological systems?
Because ΔG°′ assumes 1M conditions, while ΔG reflects actual cellular concentration conditions, which vary dramatically.
What does “irreversible step” mean?
A step with a large negative ΔG that proceeds strongly in one direction under cellular conditions.
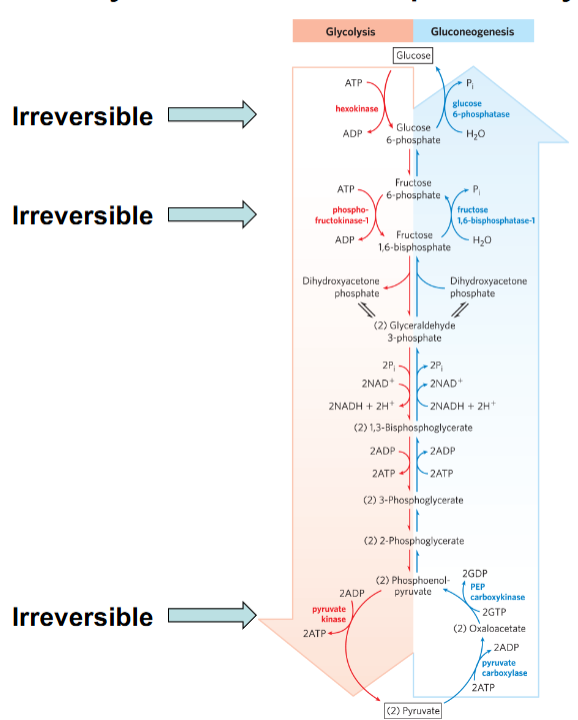
Which enzymes control irreversible glycolytic steps?
Hexokinase (Glucose → G6P)
Phosphofructokinase-1 (F6P → F1,6BP)
Pyruvate kinase (PEP → pyruvate)
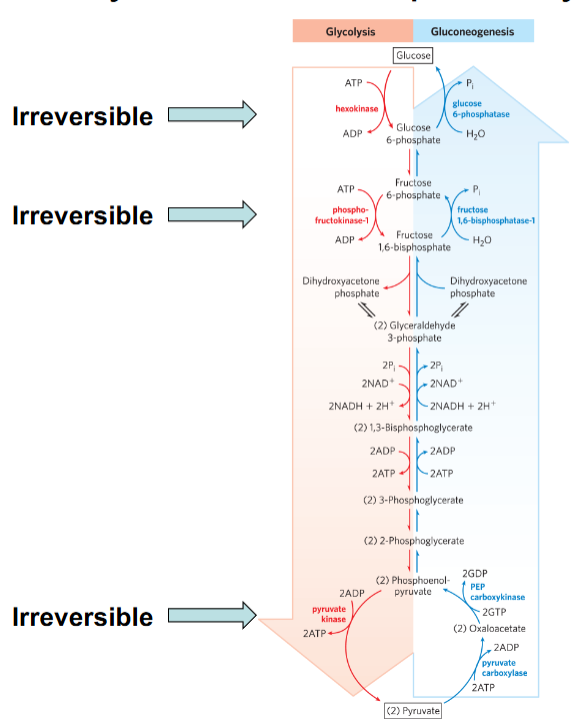
Why must glycolysis and gluconeogenesis use different enzymes at irreversible steps?
Because reversing a large negative ΔG step requires a different reaction mechanism.
Why are not all steps in a pathway required to be favorable?
Only key irreversible steps anchor pathway direction. Near-equilibrium steps adjust based on Q.
Why do irreversible steps make good regulatory points?
They commit metabolites to the pathway and respond strongly to allosteric regulation.
What does “additive free energies” mean?
For sequential reactions A→B→C
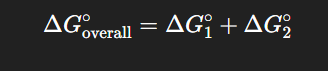
What does additivity allow?
The ability to predict the overall energetics of multi-step pathways.
What is the significance of B being an intermediate?
B does not accumulate; its concentration is determined by steady-state flux.