L5 Gas exchange (in Alveoli and Tissues) and Transport
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Gas exchange diagram
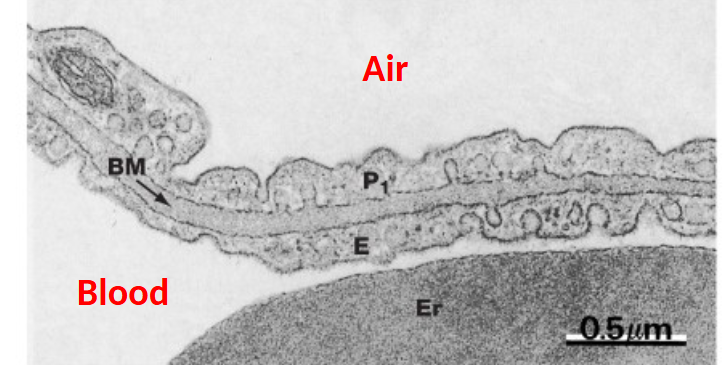
Lung perfused with Blood Prior to fixation - reveals capillaries
Air/Blood barrier
How do we make things move?
Apply force
Forces acting on gas molecules come from atmospheric pressure and heat
If a force is greater than resistance, the object will move.
Partial pressure
Atmospheric (Aka Barometric) pressure at sea level + 760mmHg
O2 = 21%
CO2 = 0.04%
N = 78.96%
PO2 = 760 * 0.21 = 159.6 mmHg
PCO2 = 760 * 0.0004 = 0.3 mmHg
PN2 = 760 × 0.7896 = 600.1 mmHg
What else other than gas is present in the air in your lungs?
Water vapour pressure
In any gas mix in direct contact with water (or other aqueous solution) there is water vapour present i.e. water in gaseous form.
The max partial pressure of water is a function of temperature alone.
At 37*C PH20 = 47 mmHg
Effect of water vapour on partial pressures
PH2O is a constant at body temperature (37*C) and equal to 47 mmHg, thus in a mixture of gases saturated with water vapour
PO2 = (PB -- 47) * FO2
Therefore for room air humidified during inspiration
PO2 = (760 - 47) * 0,21 = 149.7 mmHg
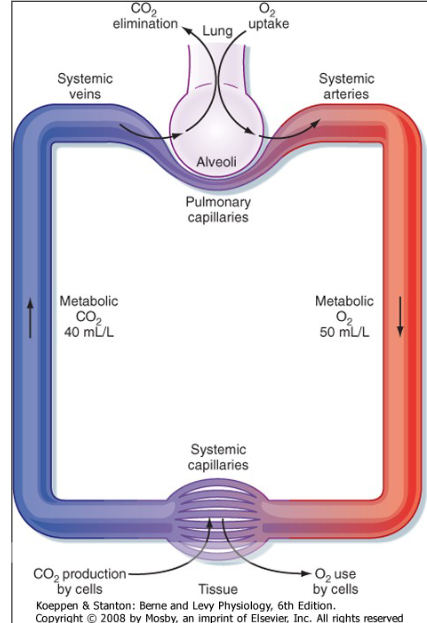
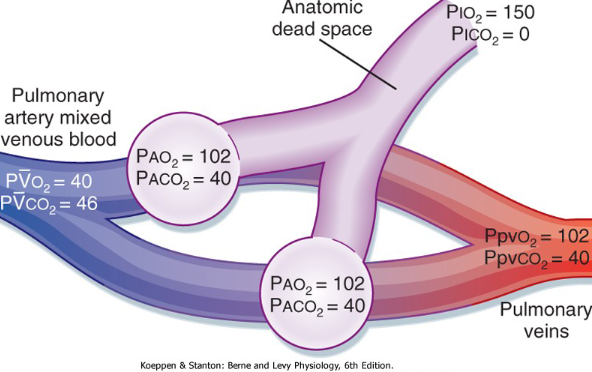
Gas exchange in Lungs
Oxygen partial pressure gradient drives oxygen from the alveolar air into the blood.
Oxygen diffuses through alveolar wall into blood
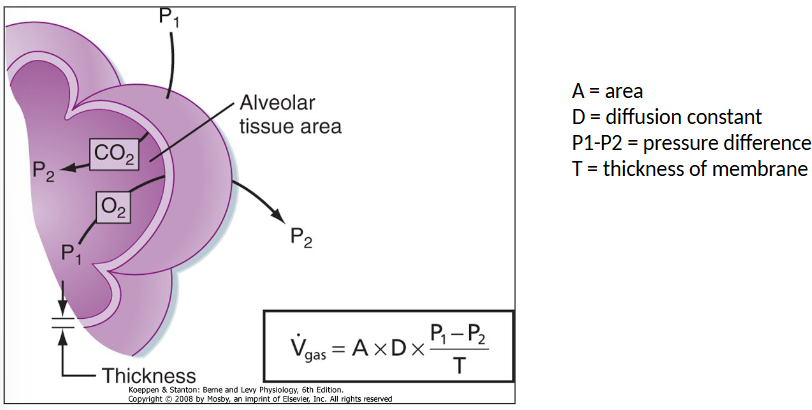
Rate of diffusion is related to:
Surface area
Pressure gradient
Solubility
Thickness of barrier
Fick’s law (rate of diffusion)
There is a capillary there but no shown in diagram
A = Area
D = Diffusion constant
P1-P2 = pressure difference
T = thickness of membrane
Ventilation (V) - Perfusion (Q) matching
Air coming in is 150
Going down to Alveoli
O2 is 102 and CO2 is 40
→ time to search it up in YT
O2 transport in Blood
Dissolved in physical solution
Bound to Haemoglobin (Hb)
Partial pressure of a Gas in a liquid
Exposure of liquid to air gas dissolves in solution
Equilibrium established when rate of entry of gas molecules into solution equal rate of exit
Partial pressure of gas in liquid is then equal to that in the aur with which is in equilibrium.
Total amount of Gas in solution depends on solubility of Gas in solution.
Gases in solution (Henry’s law)
The amount of gas dissolved in a solution is directly proportional to the partial pressure of the gas in the solution
Conc gas = S * Pgas
Where S is constant indicating the solubility of the gas in the solution (solubility coefficient).
S differs markedly between gases.
Partial pressure of oxygen in the lung
Atmomspheric PO2 = 160mmHg
Trachea/Bronchus/Bronchioles
Add heat (gas expands)
Add humidity (gas diluted)
Some oxygen consumed by Epithelial cells
Alveolar PO2(PAlv) approx. 100mmHg
Dissolved O2
Amount of O2 that can dissolve in blood is proportional to the PO2 (Henrys Law)
-0.003ml O2/100mL blood for each mmHg PO2
At normal Part O2 of 100mnnHg
-3ml O2/litre of blood
At rest
cardiac output of 5 L/min
total O2 delivered to tissue 15 ml/min
However resting tissue O2 consumption is approximately 250 ml/min
During exercise
Cardiac output of 30L/min
Total O2 delivered to tissue 90 ml/min
However exercising tissue O2 consumption is approx 3000 ml/min
Haemoglobin
Haem is an iron-porphyrin compound
Joined to the protein globin
A tetramer consisting of 4 polypeptide chains
two alpha and two beta polypeptide chains
four haem groups, each bound to an alpha or beta
Each haem group contains a porphyrin ring and a ferrous atom (Fe++) which can bind reversibly with one O2.
In deoxyHB, globin chains are tightly bound (electrostatic bonds) in a tense conformation (T) relatively low affinity for O2
Binding of O2 breaks these bonds, exposes remaining O2 binding sites leading to a relaxed conformation (R)
R conformation has an affinity for O2 which is approximately 500 times that of T conformation
Oxygen capacity
Max amount of O2 that can be combined with the Hb is called the O2 capacity
1.39ml O2/g Hb
15g of Hb/100ml normal blood
O2 capacity is about (15×1.49)
20.8ml O2/100ml b
O2 delivery - normal resting state
Consider normal Hb15g/100ml blood, then arterial concentration of O2.
(1.39 × 15) + 0.003*PO2
Giving 20.853ml O2/100ml blood
At normal cardiac output of 5 L/min, this gives O2 delivery to the tissues of approximately 1042 ml/min
Resting tissue O2 requirement approximately 250 ml/min
Large reserve
The problem of ‘‘designing’’ Hb
In the lung a molecule with a very high affinity for O2 is best to ensure maximum O2 loading
But in the tissues optimum molecule has a low affinity for O2 to allow maximum unloading
Make a molecule that change its behaviour
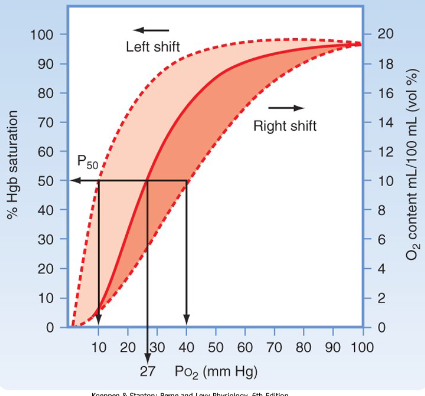
Oxygen Dissociation Curve
O2 forms an easily reversible combination with haemoglobin in RBC
O2 + Hb → ← HbO2
Advantages of O2 dissociation curve
Flat upper portion
Even if PO2 in alveolar gas falls, loading of O2 not greatly affected
Steep lower portion
Peripheral tissues can withdraw large amounts of O2 for only a small drop in capillary PO2
Maintenance of blood PO2 assists diffusion of O2 into tissue cell
Going from a high to low concentration of O2 is what’s happening in the diagram.
O2 unloading tissues
In tissues O2, unloading from Hb promoted by
decreased PO2
increased diphosphoglycerate (DPG) in RBC → by-product of metabolism
increased PCO2
increased H+
increased temperature
Denatures bond between Hb and O2
When compared to the alveoli i.e. more unloading of O2 at a give PO2
These conditions exemplified by exercising muscle
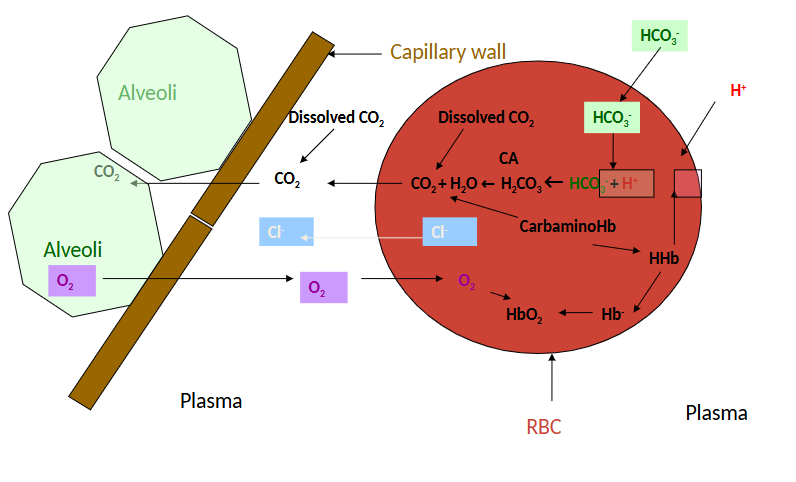
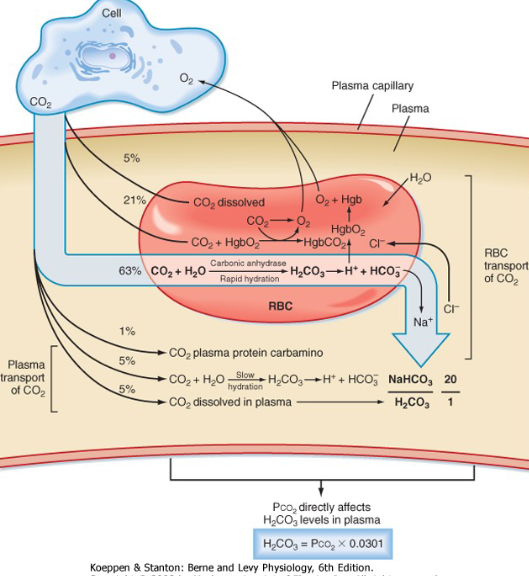
Co2 transport in Blood
CO2 transported in three forms
Dissolved CO2
HCO3
In combination with proteins as Carbamino compounds
Dissolved CO2
CO2 in solution according to Henry’s law
Solubility coefficient for O2 is 0.00314
Solubility coefficient for CO2 is 0.0746
CO2 is approximately 24 times more soluble than O2
10% of CO2 excreted in the lung is carried in mixed venous blood in dissolved form.
CO2 as Bicarbonate
More acidic if you hold your breath…
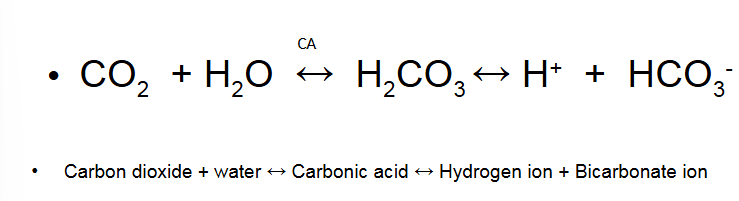
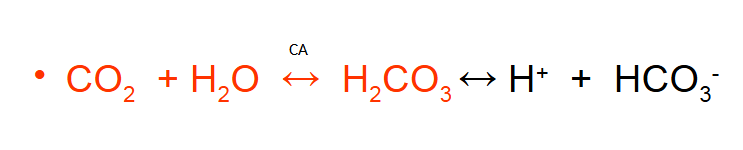
CO2 transported as Bicarbonate
First reaction is very slow in plasm but rapid when catalysed by carbonic anhydrase (CA) in RBC
Endothelium of pulmonary circulation has CA
Dissociation of H2CO3 is rapid in the absence of enzyme
60% of CO2 excreted in the lung carried in mixed venous blood as HCO3
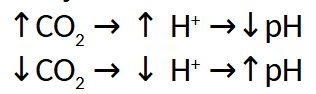
CO2 as Carbamino Compounds
Blood proteins that bind CO2 are called carbamino compounds
Globin portion of Hb most important protein (>95% of protein in RBC)
Many CO2 molecules can bind to a single Hb molecule
Hb.NH2 + CO2 ←→ Hb.NHCOOH
Reversibly produces carbaminohaemoglobin
Rapid reaction in the absence of enzyme
30% of CO2 excreted in the lung carried in mixed venous blood as carbamino form
Forms in which CO2 transported
In arterial blood
5% carried as dissolved CO2
90% carried as HCO3
5% carried as carbamino
Of the CO2 that is excreted into lung
10% carried as dissolved CO2
60% carried as HCO2
30% carried as carbamino
Hb-O2 Dissociation Curve
Amount of O2 carried by increases rapidly up to about 50mmHg
Curve becomes flatter after that
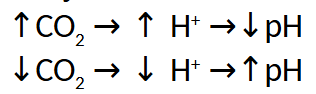
Summary of Effects in Tissues
Summary of Effects in Lungs