PH 370 Exam 2
1/121
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
122 Terms
describe aging
decrease in immune function
decrease in T cells, takes longer to destroy non self cells, vax less effective
what are the ways in which our immune system can fail us
aging, chronic inflamm diseases, immunodeficiency diseases, allergy/hypersensitivity, autoimmunity, cancers
describe immunodeficiency diseases
HIV/AIDS (HIV kills helper T cells), suseptible immune system
describe allergy/hypersensitivity
response to harmless antigen
describe autoimmunity
indivs develop autoantibodies to their own tissues/self antigens
describe the cost of inflammation
inflamm mechs = response to stimuli that disrupts homeostasis in a severe way
high benefit and high cost
cost = interfere with normal function and risk of tissue death, later in life
benefits = early in life
chronic inflamm —> many types of diseases
describe pathogens and the immune system
pathogens work hard to trick the immune system
antigenic variation = multiple variations of an antigen
latency = period of inactivity of antigen
use immune system cells as hosts
describe antigenic variation
alters surface proteins to avoid immune response
multiple variations of an antigen
used to trick immune system
antigenic shift vs. antigenic drift
shift = abrupt, major change in virus resulting in new proteins to be produced
drift = small changes in the genes of viruses that happen continually over time
describe immunoglobins
immunoglobins = antibodies (our adaptive immune system wants to remember antigens)
IgG = 75%, toxins viruses bacteria
IgA = 15%, mucous membrane structures
IgM = 10%, activates inflammation
IgD = 0.2%, bound to B cells
IgE = 0.004%, histamine present —> inflamm
challenges = immunodeficiency, hypersensitivity, autoimmunity
describe immunodeficiency
most severe type of immune suppression
primary = caused by inherited or genetic defects in the cells and tissues of the immune system
secondary = immune system is compromised due to an environmental or external factor
describe PIDD
selective IgA-deficiency
IgA protects mucous membrane lined tissues
undetectable levels of IgA, but normal levels of other immunoglobins
common, 1/300
pheno varies from no symptoms to severe illness
recurrent ear infections, lung infections, requires antibiotics, high prev in pops with autoimmune diseases and allergies
treatment of symptoms only
IgA replacement is NOT feasible
describe secondary immunodeficiency diseases (???)
caused by anything that weakens immune system (diet, stress, sleep dep, HIV/AIDS, cancer, environ, viruses, immunosuppressive treatments, age, burns, other diseases)
examples = immune system cancers, hepatitis, AIDs
how is a diagnosis of immunodeficiency disorders made?
medical history, physical exam, blood work
describe allergy/hypersensitivity
disorders that result from excessive immune responses to harmless antigens
types = enviorn, proteins in meds, insect stings
type 1, type 2, type 3, and 4 hypersensitivity
describe type 1 hypersensitivity
most common, immediate symptoms
triggers IgE response → release histamine → inflammation
allergens can cause anaphylaxis
constriction of blood vessels → low bp, rapid weak pulse, hives, vomiting
treat with antihistamines, epinephrine, corticosteroids
diagnosed by skin prick test/intradermal test, skin patch test, blood measurement of IgE, elimination diet
why is there a rise in food allergy prevalence
hygiene hypothesis = less biodiversity in orgs found in gut, system not challenged, need exposure to allergens earlier, overreporting
eggs, milk, wheat, soy, peanuts, tree nuts
describe type 2 hypersensitivity
cytotoxic: IgM or IgG mediated destruction of cells
in developing fetus, can result in isoimmunization
hemolytic disease of the fetus and newborn by maternal IgG alloantibodies which target paternally inherited
antigensdestroys RBCs
ex. Rh disease, ABO incompatability
define isoimmunization
also known as Rh sensitization or hemolytic disease of the fetus, is a condition that occurs when a pregnant woman’s immune system attacks the baby’s blood cells
describe type 3 hypersensitivity
soluble mass of immune complexes forms in blood, deposit in tissues and vessels —> inflammation
ex.) glomerulonephritis (inflamm kidney)
describe type 4 hypersensitivity
delayed hypersensitivity, activation of T cells, skin reaction
ex.) poison ivy, tuberculin skin tests
describe autoimmunity
when immune system ability to distinguish self vs. non-self fails or is uncontrollable: occurs in 2 ways
recognizes self antigens to attack
overzealous response to chronic infection
can be focused on specific organ or be systemic (grave’s = thyroid; lupus = body), often has familial tendency
systemic lupus erthematosus (SLE)
autoantibodies targeted against cell membrane, cytoplasm, and cell nucleus
common in women
includes periods of exacerbations and quinescence
diagnosis upon american college of rheumatology criteria
how many autoimmune disorders are there?
a LOT
celiac disease, RA, sjogrens, multiple sclerosis, ankylosing spondylitis, type 1 diabetes, vasculitis
what is the conundrum with the immune system?
our immune syst protects us from disease, but dysfunction of the immune syst can cause disease
describe the burden of infectious diseases
more common in low and middle income countries
90% conc on small subset of diseases: low respiratory diseases, diarrheal, HIV/AIDs, TB, malaria, measles
pathogenic microorganisms
pathogens = disease causing microorgs that grow around body triggering signs/symptoms
small % of microorgs in nature are virulent in humans (few have the ability to cause disease)
epidemiologic triad
host
the who (org thats harbouring the patho)
environment
the where
agent
the what (microbe causing disease)
center of triangle = vector = the living agent that carries pathogen
communicable diseases
diseases with human vectors
what do we want to know to prevent infectious disease
infectious period, latent period, subclinical infectious period, preclinical phase
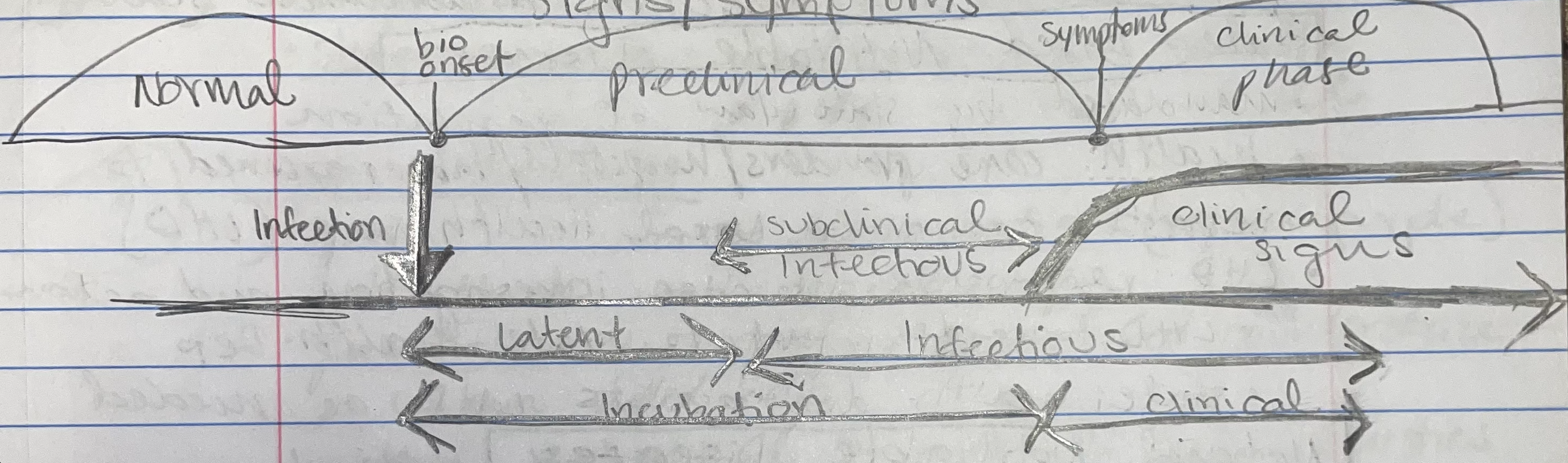
infectious period (period of contagion)
time interval when an infected host can transmit to susceptible hosts (highly variable across diff hosts)
latent period
time interval from infection to onset of infectious period
subclinical infectious period
time period from start of infectiousness to onset of symptoms
preclinical phase
incubation period in infectious disease natural history terms: interval from infection to onset of signs/symptoms
horiz vs. vertical transmission
horizontal = transmission from reservoir to sus human
vertical = one gen → next gen
direct vs. indirect transmission
direct = indiv infected thru direct contact with reservoir (including droplet transmission)
indirect = pathogen can live outside host before infecting another indiv
sometimes on fomites (contaminated inatimate object)
fecal-oral transmission
describe vector transmission
pathogen moves from one reservoir to indiv through another organism
insect bite
what is a nosocomial infection?
hospital-acquired (due in part to vulnerability of host)
endemic
normal f of disease w respect to time, pop, geo
sporadic
occasional disease thats unexpected but doesn’t prompt further cases
epidemic & pandemic
epidemic = occurence of disease in excess of normal expectency with respect to TPG
pandemic = an epidemic on a world wide scale
State-Based Notifiable Diseases
Each state might have its own special list of diseases that they need to know about because they might be more common or important in that state. So, doctors in each state tell the state health department about these diseases so they can protect everyone in that state.
local health department responsible for case investigation and action, LHD forwards it to state health dep, state assists LHD as needed
National Notifiable Diseases
These are diseases that doctors and hospitals in the U.S. have to tell a the CDC about. The CDC collects this information and shares it to make sure everyone across the whole country is healthy and safe.
reporting is voluntary
cases must meet national surveillance case definition
some are non-infectious such as cancer, CO poisoning, lead, pesticide
NNDSS = nationally notifiable disease surveillance system
International Notifiable Diseases
These are diseases that could spread quickly around the world or are very dangerous, so countries have to tell big health organizations, like the World Health Organization (WHO), about them. all WHO members must report PHEIC (internationally concerning events).
can spread from any major city on any continent in <36 hours
international health regs = legally binding agreement of 196 countries requiring all to have ability to assess, report, respond to PH events (only ~1/3 countries)
countries must assess PH risks <48hrs, report to WHO if notifiable in <24hrs
smallpox, new subtype human influenza, SARS, potential events
classes of pathogens
bacteria, viruses, protozoa, fungi, helminths, prions
bacteria
single celled orgs (cell walls, no nuc, no organelles)
shapes = coccus, bacillus, vibrio, spirilla
gram staining
gram (+) = resist decolorization: retain purple = thick walled cells which can hang onto the color
gram (-) = decolorized and accept red counterstain = thin walled cells which couldn’t hang onto the first color
aerobic vs. anaerobic
aerobic = use oxygen to perform aerobic respiration where oxygen acts as the final electron acceptor in the electron transport chain, which produces a high yield of ATP (energy) for cellular processes.
anaerobic = doesnt require oxygen, They rely on anaerobic respiration or fermentation to produce energy, less ATP produced but more sustainable
bacteria biochemical and cultural characteristics
varying levels of bacterium’s ability to grow in environs w/ varied temp and acidity
flagella
hair like processes covering bacterias surface and give motility
endospores
spherical structures produced by bacterial cells that can survive extreme conditions
describe staphylococci
normal inhabitants of skin and nasal cavity
type of bacteria, generally not pathogenic, but some are very virulent
nonpathogenic ones don’t hemolyze (break down) RBCs
pathogenic staphlococci: boils, skin infections, post op wound infections, systemic infections
some strains are antibiotic resistant
describe streptococci
bacterium classified on basis of serologic group and type of hemolysis of RBCs
serologic = based on antigens on cell walls
20 diff groups: important = A,B,D
hemolysis types:
Alpha = mouth/throat, no patho
Beta = complete hemolysis
Describe antibiotics
substances that destroy bacteria or inhibit their growth
act on bacteria in many diff ways: inhibit cell wall synthesis, metabolic function, protein synthesis
Describe Viruses
infectious particles with a core of genetic material wrapped in a protein coat (protein spikes, lipid envelope)
parasitic: require a host cell, don’t independently grow, metabolize, reproduce
can be latent
diff viruses target diff tissues
no cure
smallpox, rabies, common cold, ebola, flu, HIV/AIDS, SARS, herpes, polio, zika, hepatitis
latent
a virus that is present in the body but is not actively replicating and causing symptoms
treatment for viruses
no cure, but vax can confer protection
monoclonal antibodies
convalescent plasma
antiviral drugs dont destroy the pathogen
inhibit development and slow disease progression, interfere w virus rep, new virus assembly, and attachment of viruses to host cells
protozoa
single celled euk microorgs
have nuc and organelles
no viruses!
most don’t cause disease; many can destroy tissue and induce inflammation
types = amoeboids, flagellates, ciliates, sporozoans (malaria)
fungi
single or multi celled orgs within a cell wall that contain chitin (polysacc)
reproductive structures bear spores, known allergens
mycoses = fungal infections
mycoses
fungal infections
often opportunistic
can be endogenous or exogenous
classified as superficial, cutaneous, subcutaneous, systemic (deep)
History of Tuberculosis
originally spread animals → humans 100,000 y/a
many names throughout history: consumption, phyhisis, scrofula, potts disease, white plague
early disagreement about etiology:
hippocrates ~ hereditary; lungs
aristotle ~ air has smthn in it thats disease producing
Describe the discovery of Tuberculosis etiology
Robert Koch = “father of bacteriology”
developed new staining method to view pathogens under a microscope
won nobel prize in physiology in 1905
confirmed TB not hereditary
TB pathogenesis
pulmonary TB: affects lungs (85% of cases, contagious)
extrapulmonary TB: affects other sites (skin, kidney, skeleton, brain, not contagious)
describe modern day TB (1880s-1940s)
contagious and airborne infectious disease
sanatoriums = first PH step, not effective in curbing TB
curable and preventable in 1940’s with first antibiotic
no longer a significant effect to wealthy nations
TB burden
a top COD worldwide in 2015 (more than HIV/AIDS)
only 5-15% of TB infection will develop active TB
# deaths fell 1/3 between 2000-2017
90% of all TB cases are adults
male to female = 1.6:1
55% of TB patients globally have documented HIV+ test result
wide variation in case fatality ratio (5-20%)
TB transmission
direct transmission: spread when people sick with pulmonary TB expel bacteria into the air (droplets can hang in air for hours)
ppl with active, untreated TB infect 10-15 ppl/yr
Immune response to M.tuberculosis
macrophages try to contain pathogen by phaocytosis: if not killed, break out of phagocytic vesicle and multiply inside macrophage
after several phases, tubercule is formed
spread through blood stream and lymph that drain to lungs
Tubercule
tumor-like module: lumps of scar tissue where bacteria can remain dormant for decades/lifetime
Latent TB infection and TB Disease
Latent Infection:
no symptoms, doesnt feel sick
cannot spread TB bacteria to others
usually has a skin/blood test result indicating TB infection, normal chest xray and sputum smear
needs treatment for latent TB to prevent disease
Disease:
has TB symptoms, usually feels sicks
may spread TB bacteria to others
usually has a skin/blood test result indicating TB infection, may have abnormal chest xray or positive sputum smear or culture
needs treatment to treat TB disease
TB symptoms
bad cough lasting 3+ weeks, pain in chest, coughing up blood/sputum, weakness or fatigue, weight loss, no appetite, chills/fever/night sweats
TB: Latent VS. Disease
5% develop TB disease in first 2 years after infection
after first 2 years of infection, 5% risk of reactivation (developing TB disease)
risk of reactivation 10-15% per year for HIV+ patients
Broadly describe testing for TB
there are several types of tests:
testing that doesn’t initiate treatment
tests that does initiate treatment
describe tests for TB that don’t initiate treatment
tuberculin skin test: tells if youve been infected (2 visits, if + → additional testing)
blood test: tells if infected, addtnl testing required
chest xray: nonspecific, insufficient to begin treatment
describe tests that initiate treatment
sputum spear microscopy = look for presence of bacteria
koch’s method
culture methods
current standard but require a lab, many weeks
allows testing for drug susceptibility
rapid molecular tests - PCR assay
can be used on pulmonary TB and certain forms of extrapulmonary TB
better accuracy than microscopy
TB prognosis
often treatable: many treatments are old (60’s antibiotic)
without treatment, 1/3 theory
1/3 die, 1/3 self cure, 1/3 remain infectious
Treatment of drug-susceptible TB
New cases (never treated before) - category 1 regimen (85% success rate)
6 month regimen of 4 first line drugs
isoniazid, rifampicill, ethambutol and pyrazinamide
daily dosing
supplied by global TB drug facility ($40/person)
failed or interrupted treatment (60-80% success rate)
old guidlines = cat 2 regimen = 8 months of first line drugs + injectable antibiotic for 2 months (stopped in 2017 due to AMR concerns)
new guidelines = drug susceptibility testing
if not resistant, repeat cat 1 regimen
if resistant, move to MDR-TB regimen
Describe antibiotic resistance
resistant bacteria can travel: food, people, animals, international borders
3 million abx resist infections/year
antibiotics kill both good and infectious bacteria in body: nonspecific utility of antibiotics → body may be unable to fight these resistant bacteria
most susceptible bacteria die first, leaving resistant bacteria → multiply to create a more hearty disease
What are bacterial resistant mechanisms / defense strategies?
restrict access of the antibiotic
get rid of the antibiotic
change or destroy the antibiotic
bypass the effects of the antibiotic
change the targets of the antibiotic
treatment for drug-resistant TB
requires use of second line drugs ($, more toxic, long), regimen shortened to 9-12 months (decreased cost, $1000)
every TB antibiotic has 1+ resistant strain
not all MDR-TB is curable: 52% cure rate, 28% cure rate for extensively resistant TB (XDR-TB)
only ¼ needing MDR-TB treatment were enrolled in it
describe DOTS
DOTS = directly observable therapy
clinical intervention + community based PH
monitor medication intake; monitoring done by nurse or health dep outreach member
increases liklihood of completing therapy
90% on DOT vs 60% self admin
patient-centered approach: removing barriers, education, incentives
Vaccines for TB
Bacille Calmette-Guerin (BCG) vaccine
developed in 1921, can prevent TB in kids, widely used, given to all infants in high burden areas
no vax effective in preventing TB in adults
13 vaxes in phase 1,2,or 3
describe TB prevention
treatment of latent TB prevention, infection control to prevent transmission, vax of children with BCG vaccine in high risk areas/indivis
describe the WHO end TB strategy
goal by 2030, written in 2015:
80% drop in new TB cases, 90% drop in TB deaths, 100% of TB affected families protected by catastrophic costs
43m lives saved, 47% decrease mortality rate, 32% decline in HIV related TB deaths, MDR-TB treatment increased
What are the Pillars of ending TB
integrated patient centered care and prevention
bold policies and supportive systems
intensified research and innovation (vaxes tools)
Describe the financing of TB
$8.3 billion dollars spent in 2016, $1.4 billion funding gap
low income countries rely on international donors for 90% funds
funding gap for research = $1.3 billion
describe the most common influenza pandemic
H1N1 = recurring through time
many major influenza subtypes since 1885
very common because it is unstable and easy to recombinate
The flu vs. influenza (seasonal)
“the flu” = the public description of cold like symptoms; nonspecific symptoms
“ILI” = influenza-like-illness
may or may not be caused by influenza virus
influenza = substantial morbidity & mortality, predictable and persistent (yr-to-yr & seasonally), has an infrastructure for prevention
Describe respiratory viruses
RSV, Flu A, Flu B, Corona, Adeno, Paraflu
PH implications of influenza vs. other = significant because it mutates quickly
Influenza Symptoms
start 2 days (1-4) after infection
systemic = fever, headache, myalgia, malaise
respiratory tract symptoms
possibility for superinfection: secondary bacterial pnemonia
large contributer to morbidity/mortality of influenza
possible resistance
Influenza transmission
direct: inhalation of aerosolized viral particles
indirect: contacting a contaminated surface
virus can survive for several hours on fomites
avian influenza - water and feces
Influenza etiology
Orthomyxoviruses: 3 species classified by core proteins
Influenza A: most signif, highly pathogenic in humans, very virulent
seasonal outbreaks peaking during winter
affects many mammals and birds
Influenza B: human illness, less common, less pathogenic
seasonal outbreaks
humans and seals
Influenza C: mild disease, no seasonal variation
humans and pigs
Influenza Pathogen
Transmitted through droplet contact from coughing and sneezing; most efficient when virus reaches lower respiratory tract
enveloped RNA, segemented genome 80-120nm
Influenza type A: enveloped by a protein based core, Neuraminidase (NA) on outside, Hemagglutinin (HA) on outside, M2 ion channel protein
B has diff channel ion protein
Describe Hemagglutinin and Neuraminidase
viral proteins on surface of influenza virus
Hemagglutinin binds the virus to the cell being infected
Neuraminidase is the protein enzyme that helps the virus enter cell walls
How is Influenza named
viral proteins that both identify themselves and are the mechanism by which that pathogen can enter host cells
H1N1, H2N2, etc.
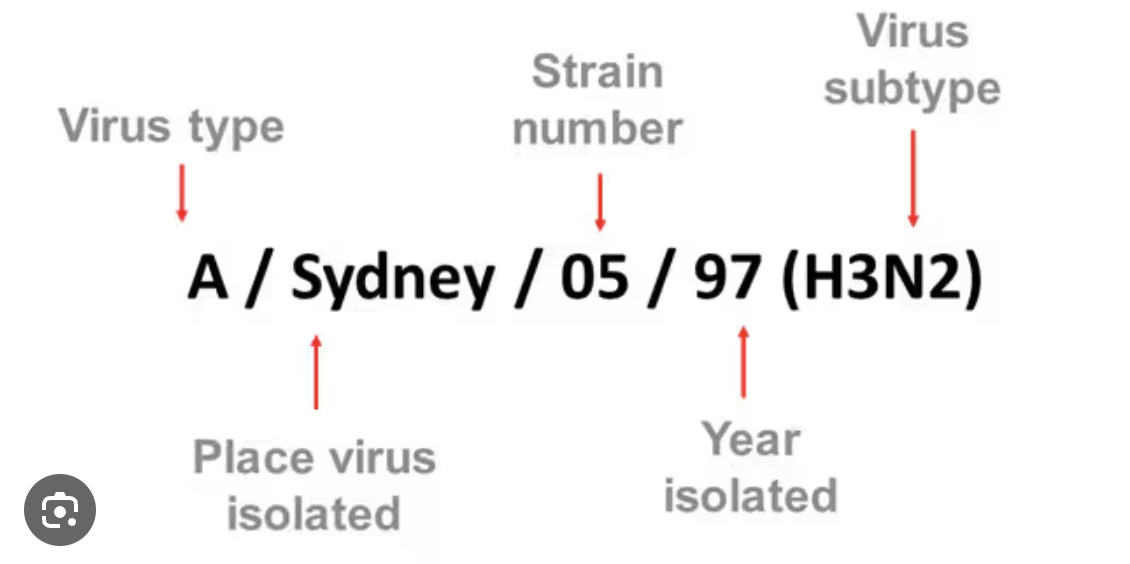
WHO/CDC naming convention:
antigenic type, host of origin if not human (determined by sampling), geographical origin, strain number, year of isolation, & for influenza A designate H and N antigen description
6 H types & 3 N types contribute to disease in humans
A / Perth / 16 / 2009 (H3-N2)
Attachment and Entry of Influenza
wants to get into cell to release its viral RNA so that it can use cells machinery to rep (viruses are not cells, theyre just particles with RNA)
binds through hemagglutinin
binds to receptors on cells
secrete Neuraminidases to break down cell structure
create pit allowing virus to go deeper into cytosol
adopted in, move enveloped in membrane
fuse, burst, use cell for RNA
Describe the age specific morbidity and mortality of influenza
RIR = relative illness ratio (% sick in age group : & pop in age group)
worst symptoms and RIR in younger individuals
RMR = relative mortality ratio
ratio >1 indicates an excessive risk
older adults affected more by superinfections
Influenza Seasonality
A and B peak in winter months in both hemispheres, infections mapped by morbidity week (defined start of influenza surveillance szn)
24-25 szn starts oct.1
tied to bird migration patterns
Antigenic variation: influenza shift vs. drift
antigenic shift = abrupt, major changes in virus resulting in new proteins to be produced
only occurs in A because its the only one that shares recombinant species
2 subtypes of virus infect same host, exchange genetic components and new surface molecules
antigenic drift = small changes in the genes of viruses that happen continuously over time
require targeted annual vaccine
1918 influenza pandemic
young ppl affected, lasted many years, started developing natural immunity
1944 = development of inactivated flu vaccine (UM SPH dr. thomas francis jr and jonas salle)
1945=military and students; 1946=civilian
1957 influenza pandemic
first flu pandemic with a vaccine
didnt hear much about this one because able to curb # cases and core outcomes from 1 mil to 70k
developed vax in 4 months after identifying the new pathogen because they had the right tools
seasonality: knew when its coming —> start production early