ex 2 simple distillation of organic solvents
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
simple distillation
involves the process of separating two or more liquids homogenously mixed but with different boiling points (at least 20° difference)
lowest
as the distillation progresses, the concentration of the __ boiling component will steadily decrease
-
simple distilation
eventually, the temperature within the apparatus will begin to change and a pure compound can no longer be distilled
• the temperature within the apparatus will continue to increase until the boiling point of the other liquid left at the distilling flask
• when the temperature again stabilizes, another pure fraction of the distillate can be collected (fractional distillation)
-
distillation set up
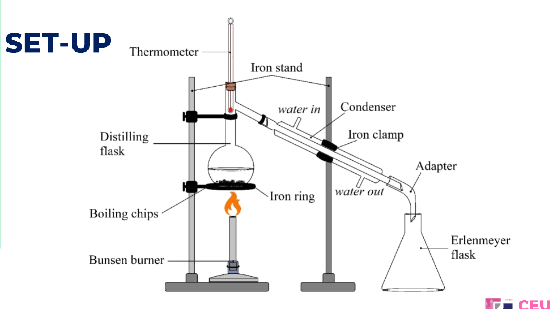
boiling chips
small, insoluble, porous stones made of calcium carbonate or silicon carbide
calcium carbonate or silicon carbide
boiling chips are small, insoluble, porous stones made of __
boiling chips
have pores inside which provide cavities both to trap air and to provide spaces where bubbles of solvent vapor can form. these bubbles ensure even boiling and prevent bumping and boiling over and loss of the solution
right below the side arm
the bulb of the thermometer should be placed __ of the distillation flask
-
simple distillationn process

lowering the pressure
the application of distillation is limited to a certain extent due to the of decomposition some organic compounds when they are distilled at normal atmospheric pressure
this problem can be resolved by __, thus, lowering the boiling point of the substance
35
theoretical boiling point of diethyl ether
77
theoretical boiling point of ethyl acetate
40
theoretical boiling point of dichloromethane
78
theoretical boiling point of ethanol
56
theoretical boiling point of acetone
117
theoretical boiling point of n-butanol