Solvent Extraction
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
If ether and water are mixed in equal volumes, the top layer will be:
Ether
In this experiment, what is the mobile phase used for the TLC analysis? Choose the best answer.
3:7 Ether:Chloroform Mixture
In this experiment, what is the stationary phase used for the TLC analysis? Choose the best answer.
silica gel
What is the purpose of adding sodium sulfate (Na2SO4)?
Drying the organic layer
Solvent extraction requires the use of two solvents that are immiscible. Using the list below, pick the best solvent to use for a liquid-liquid solvent extraction with water.
methylene chloride
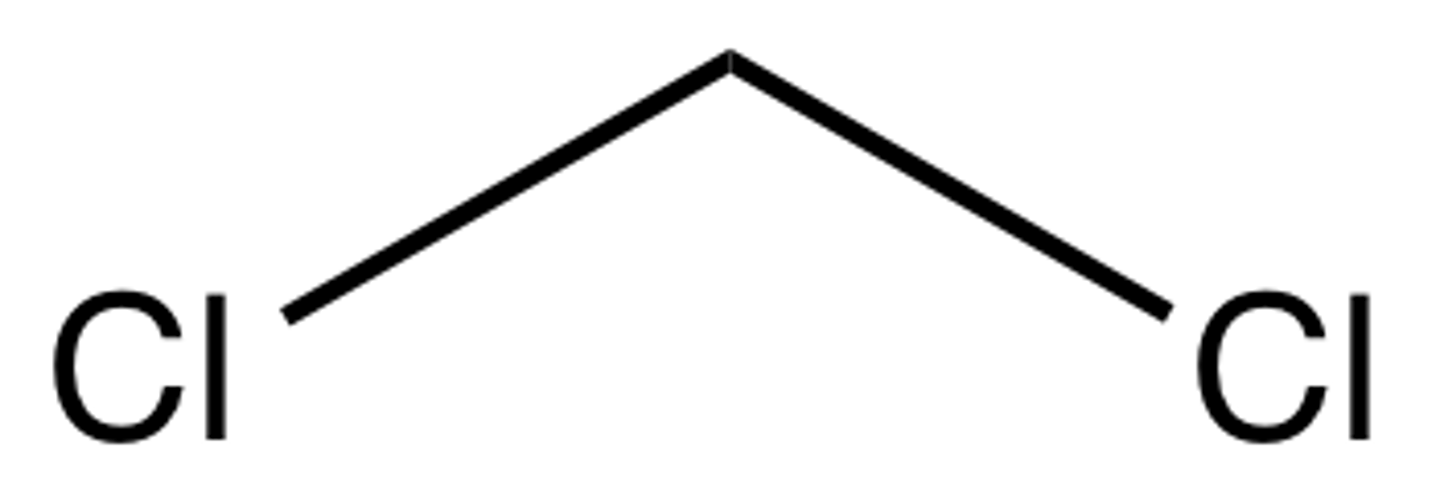
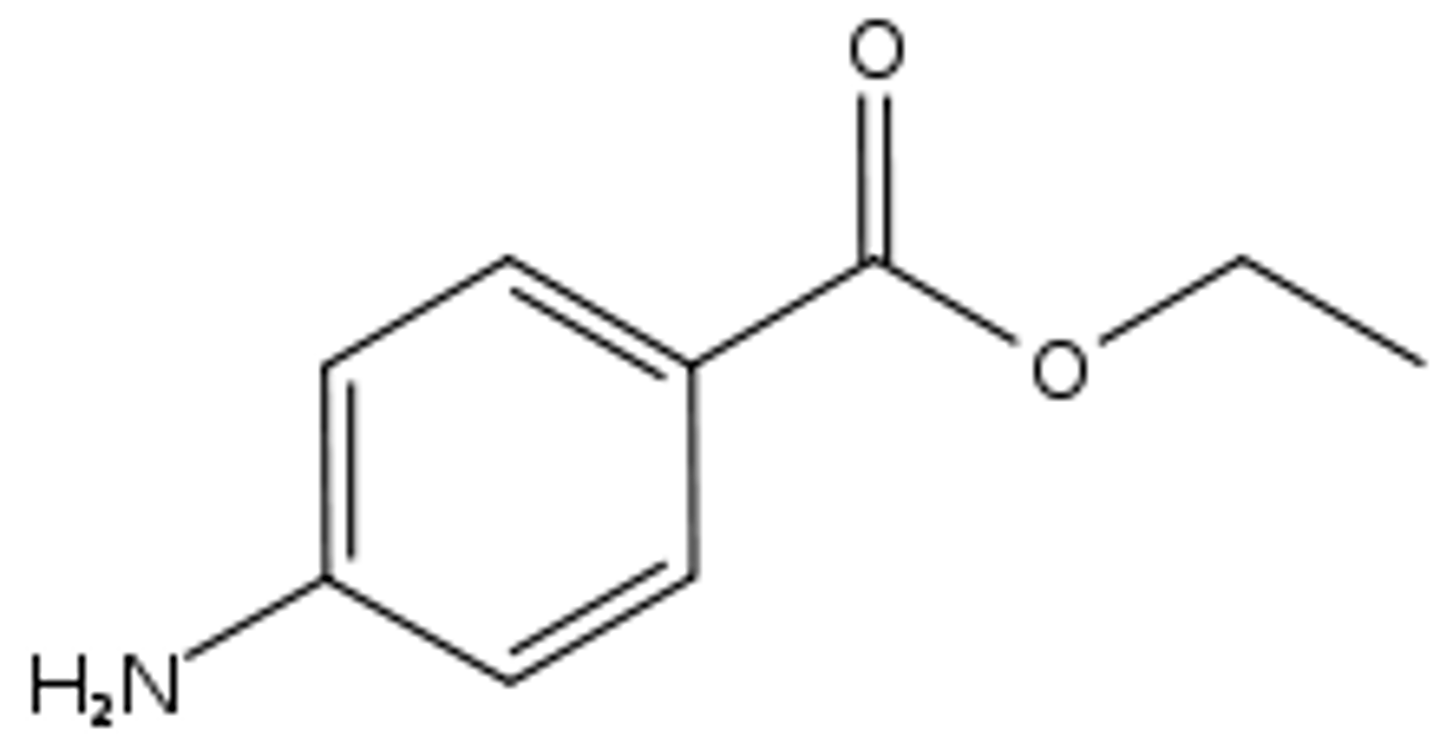
If a solid mixture of the three aromatic compounds shown below is placed in 3 M HCl, which is likely to dissolve?
C- ethyl 4-aminobenzoate (the base component)
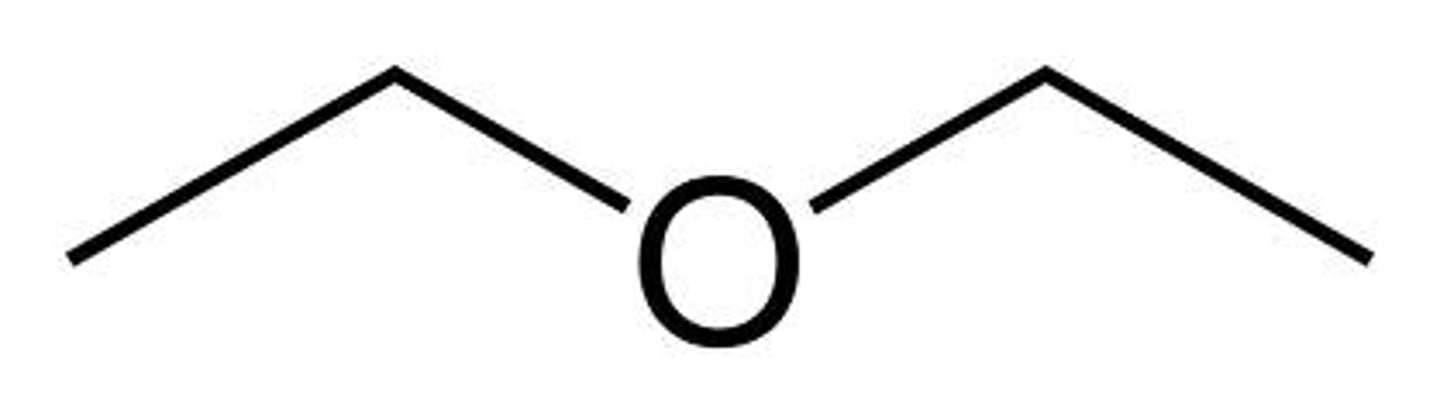
Pick the solvent below that can dissolve all three aromatic compounds of the mixture which is used in this experiment.
diethyl ether
Where would you dispose TLC plates after you are done with the experiment?
Solid hazardous chemical waste container
T/F: SiO2 is a Lewis Base.
false
T/F: Glucose is more soluble in hexane than water.
false
T/F: Compounds that interact more strongly with silica gel will travel more quickly on a TLC plate.
false
T/F: A UV or ultraviolet light/lamp will be used to detect compounds once they are spotted and run on a TLC plate.
true
Suppose you are working with a NaOH stock solution but you need a solution with a lower concentration for your experiment.
Calculate the volume (in mL) of the 1.031 M stock NaOH solution needed to prepare 250.0 mL of 0.1403 M dilute NaOH solution.
M1V1=M2V2
34.02 mL
In chemistry, TLC stands for ______________________. The purpose of TLC is to _______________________.
Thin Layer Chromatography, separate components of a mixture