Lewis Dot and Covalent Bonds
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What are valence electrons?
Electrons in the outermost shell of an atom
What is a lewis dot structure of oxygen?
Lewis Dot only shows the valence electrons. If its a single oxygen, it has 6 valence electrons. If its O2 and shared, each has 4 and 2 shared which are represented by lines like so.
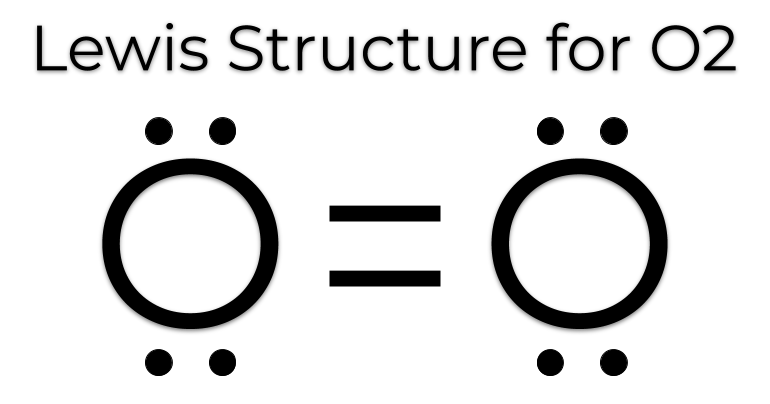
What is the octet rule?
Atoms aim to have 8 valence electrons to become stable. They will lose/gain or share to achieve this.
If atoms share electrons to satisfy the octet rule it is called a _______
Covalent Bond
What are lone pairs?
Pairs of valence electrons not involved in bonding
If a molecule like water has one atom of one element and two of another, where do we usually find the single atom in relation to the others?
In the center.
What are double bonds?
Two atoms sharing two pairs of electrons.
Diagram a structural formula for oxygen showing the double bond
O=O
Diagram a structural formula showing Nitrogen with a triple bond
N=-N. Three electrons shared.
How does the strength of a triple bond compare to that of double and single bonds?
Triple bond is the strongest.
What does the term resonance mean and diagram resonance form of ozone.
Resonance means electrons are shared in multiple ways rather than just one. O-O=O (Ozone)
Diagram the reactions of oxygen and ozone with UV radiation
O3 → O2 + O when UV hits O3.
What is a steady state?
A system that stays constant over time, even with ongoing processes.