Straumanis Memorization
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
Strong Acids
HCl, HBr, HI, HNO3, H2SO4, OH3
Very Good Leaving Groups
RSO-, I-
Good Leaving Groups
Cl-, Br-, H2O
(Very) good leaving groups are...
very weak bases
Poor leaving groups are...
strong bases
Poor Leaving Groups
F-, RO-, R2N-, R3C-
Very good nucleophiles
RS-, NC-, I-, PR3, R3C-, R2N-, RC---C-, RO-
Good nucleophiles
Br-, R2S, NR3, Cl-, RCO2-, N3-
(very) good nucleophiles are...
good bases
poor nucleophiles
F-
HCO3-
R2O (water, alcohol, or ether)
poor nucleophiles are...
weak bases
examples of soft nucleophiles (also are good leaving groups)
I-, Br-, PR3, R2S, & Cl-
Nucleophicity increases...
as you go down the periodic table
13.1: R-CH2-OH => R-CH2-X
reagents: HX (X= Cl, Br, or I)
OH is replaced with an X
13.2: R-CH2-OH => R-CH2-Cl
reagents: SOCl3
OH is replaced with a Cl
13.3: R-CH2-OH => R-CH2-Br
reagents: PBr3
OH is replaced with a Br
13.4a: R-CH2-LG => R-CH2-Nuc
reagent: any nucleophile
LG is replaced with nucleophile
13.4b: R-CH2-LG => R-CH2-OH
reagents: NaOH or KOH
LG replaced with OH
13.4c: R-CH2-LG => R-CH2-OR
reagents: Na (metal; same one u use for alkyne to alkene reduction trans) + ROH (an alcohol) = NaOR
LG replaced with OR
13.4d: R-C---C-H => R-C---C-CH2-R
reagents = 1) strong base so NaNH2
2) R-CH2-LG
H is replaced with everything in step 2 except the leaving group
8.1: Markov Addition of H & OH
reagents: 1) Hg(OAc)2, H2O & THF (solvent)
2) NaBH4
H2O creates OH and it gets added onto the Markov. carbon. Remember the double bond gets broken.
8.2: Markov Addition of H & OR
reagents: 1) Hg(OAc)2, HOR & THF (solvent)
2) NaBH4
OR gets added on Markov. carbon.
8.3 Anti-Markov. Addition of H & OH
reagents: 1) BH3 & THF
2) NaOH, H2O2, H2O
Double bond gets broken & OH is added to Anti-Markov carbon.
8.4: Anti-Markov. Addition of H & Br
reagents: HBr & peroxides like light & heat
Br is an exception from the usual HX unless its no peroxides.
Double bond breaks & Br is added to the Anti-Markov. carbon.
Needs more heat/energy to add to Anti-Markov.
8.5: Markov. Addition of H & X (X = Cl, Br, or I)
reagents: HX & no peroxides (dark & cold)
X is added to Markov. carbon. HX is a strong acid & acids prefer Markov.
9.1: Addition of Br2 to an Alkene (a ring)
Reagents: Br2
Double bond in a ring breaks. Creates 2 Br additions.
Has trans configuration.
9.2: Oxidation of an Alkene to an Epoxide
Important points:
reagent - MCPBA = a peroxy (carboxylic acid with an addition O)
Double bond between two C's break & creates a triangle with an O in the middle.
9.3: Anti-Markov. Epoxide Ring Opening (Basic Conditions)
reagents: 1) strong base (ex: RO-)
2) dilute acid (H gets added to O-)
The base causes the triangle to split. Only one side gets the O- & the other gets the H & the base. The acid job is to make the O- to a neutral value.
9.4: Markov. Epoxide Ring Opening (Acidic Conditions)
reagents: H+ & HOR
9.5: Markov. Addition of Br & OR to an Alkene
reagents: Br2 & HOR
OR ends up on the more subs. carbon & Br on Anti-Markov.
10.1: Hydroboration-Oxidation
reagents: 1) BH3 & THF
2) NaOH, H2O2, H2O
Anti-Markov. cuz OH ends up on less substituted carbon.
H & OH = cis/syn
H3C & OH = trans
H2 & Pt/Pd can...
completely reduce any bond to a sigma bond. the two H's are syn of each other.
H2 / Ni2B
or
H2 / Lindlar Catalyst
alkyne => alkene in cis/syn
Na0 (metal) NH3 (ammonia)
alkyne => alkene in trans
D2
Double bond will reduce but 2 D's will form in a syn configuration touching each side of the bond.
Oxidation of 1* alcohol to aldehyde
PCC
reduces an OH => double bond O
Oxidation of 2* alcohol to ketone
KMnO4 or Na2Cr2O7/acid/H2O or CrO3/acid/H2O
reduces an OH => double bond O
10:9 Syn Addition of Two OH Groups to a PI Bond
reagents: 1)OsO4
2)H2O2 or NaHSO3
the two OH added Syn/Cis
Trans Addition of Two OH Groups to a PI Bond
acidic: 1) MCPBA
2) H3O+ & H2O
OH and methyl Markov. carbon
basic: 1) MCPBA
2)KOH
3) neutralizes with dilute acid
methyl group on other carbon
OH & a methyl shown
acid: OH is on anti-Markov. & methyl on other
that means: 1) MCPBA
2) Na + (methyl)
2) H+
base: OH is on Markov, & methyl is on anti-Markov
1) MCPBA
2) (methyl) + OH
2)H2SO4 or H+
10.10: Ozonolysis
reagents: 1) O3 2) Zn/acetic acid
cleaves double bond in half, it only oxidizes the carbon to an aldehyde under reducing conditions. if ozidizing make same product as KMNO4
10.11: Permanganate
reagents: 1) KMnO4 (hot)
2) H3O+, H2O
cleaves C=C into making 2 O=C. OH is attached to one of the sides.
11.1: Markov. Addition of H & X (X = Cl, Br, or I)
reagents: 1) HX so no peroxides
2) excess HX
goes from triple bond to sigma & creates germinal dihalide (2 X on one side Markov)
11.2 Addition of X2 (X = Cl, Br, or I)
reagents: 1) X2
2) X2 (excess)
2 X's on each side. They are trans to each other.
11.3: Markov. Addition of H2O to an Alkyne
reagents: H2O2, H2SO4, HgSo4
O added to Markov & triple bond becomes a double bond with O connected to it
11.4: Anti-Markov. Addition of H2O to an Alkyne
reagents: 1) BH3 & THF
2) NaOH, H2O2, H2O
double bonded O ends up on Anti-Markov. carbon
11.5: Deprotonation of an Alkyne
reagents: NaNH2
The H on the right of the triple bonded C disappears. The C is left with a lone pair of electrons and a - charge. Ends up creating an acetylide anion.
polar aprotic solvents
- polar solvents without an H on N, O, or X
- faster in SN2
polar protic solvents
- polar solvents with an H on N, O, or X
- faster in SN1 (since it lowers the PE of the intermediate, lowers E act)
Substitution of allylic & benzylic
Can undergo either SN2 or SN1 depending on the solvent; although, in tertiary groups, they cannot undergo SN2 (too hindered)
steric factors
the ability of groups, because of their size, to hinder access to a reaction site within a molecule
overcrowding & depends on the degree of carbocation
electronic factors
factors having to do with where the electrons are found (ex: electronegativity, resonance, hybridization, or inductive effects)
methyl leaving group
SN2 only possibility
1 degree leaving group
good nucleophile (both weak & strong bases work) => SN2
strong base & poor nuc (& hot) => E2
2 degree leaving group (or 1 degree with allylic or benzylic leaving group)
good nucleophile => weak base & polar protic solvent (cold) => SN1
good nucleophile => weak base & polar aprotic solvent => Sn2
good nucleophile => strong base & cool => SN2
poor nucleophile => weak base (& polar protic solvent hot) => E1
poor nucleophile => strong base => E2
good nucleophile => strong base & hot => E2
3 degree leaving group
weak base => cool or good nucleophiles => SN1
SN2 not possible
weak base => hot and poor nucleophile => E1
strong base => E2
Zaitsev's Rule
an elimination usually gives the most substituted alkene product.
Hoffman Rule
When an elimination reaction yields the alkene with the less substituted double bond.
15.1: Radical Chlorination of an Alkane
start: C is attached to 2 R's & H & a methyl group (CH3)
result: it creates 3 products = 1) Cl attaches to the methyl group, 2) Cl replaces the H as the fourth bond to the central C, 3) HCl
*remember why it creates more products than Br = Cl products equally divided & doesn't prefer a certain form
reagents = Cl2 & light (hv)
Synthetic Transformation 15.2: Radical Bromination of an Alkane
start: C is attached to 2 R's & H & a methyl group (CH3)
end: 2 products = 1) Br replaces the H as the fourth bond to the central C 2) HBr
*Br is more selective => tertiary bromide product is most favored
reagents:
Br2 & light OR NBS (creates Br2 & radicals quickly) & light
radical
species with an unpaired electron (result from nonpolar bond breakage)
shown with a half arrow ("single barbed" arrow)
radical potential energies (highest P.E. to lowest P.E.)
1. H
2. CH3
3. 1 degree
4. 2 degree
5. 3 degree
6. allylic
7. benzylic
8. X (halogen)
radicals in synthetic transformation:
Br2 => ???
radical initiator + HBr =>
HBr =>
look at pg 231 for visual reference
1. Anti-Markov.
2. Anti-Markov
3. Markov.
bond angles & shapes
1. count the number of electron domains
=> 4 - 109.5 degrees, 3 - 120 degrees, 2 = 180 degrees
2. Find the molecular shape (you can figure this out by knowing the electron domain & how many bonds/lone pairs in each molecule)
Linear
- 2 electron domains
- 180 degrees
- no lone pairs, all bonds
Bent (2 types)
- 3 electron domains
- 120 degrees
- 2 bond domains (the 3 bonds are usually split as 1 double bond and 1 single bond) & 1 lone pair
- 4 electron domains
- 109.5 degrees
- 2 bonds & 2 lone pairs
- common ex: water (H2O)
Trigonal planar
- 3 electron domains
- 120 degrees
- all bonds, no lone pairs
Pyramidal
- 4 electron domains
- 109.5 degrees
- 3 bonds & 1 lone pair
Tetrahedral
- 4 electron domains
- 109.5 degrees
- 4 bonds, 0 (no) lone pairs
Formal charge
group # - # lines - # dots
hybrid orbitals
sp3 => 109.5 degrees
sp2 => 120 degrees
sp => 180 degrees
Strong base
molecule with a lone pair & -1 formal charge on H, C, N, or O
phenol
10
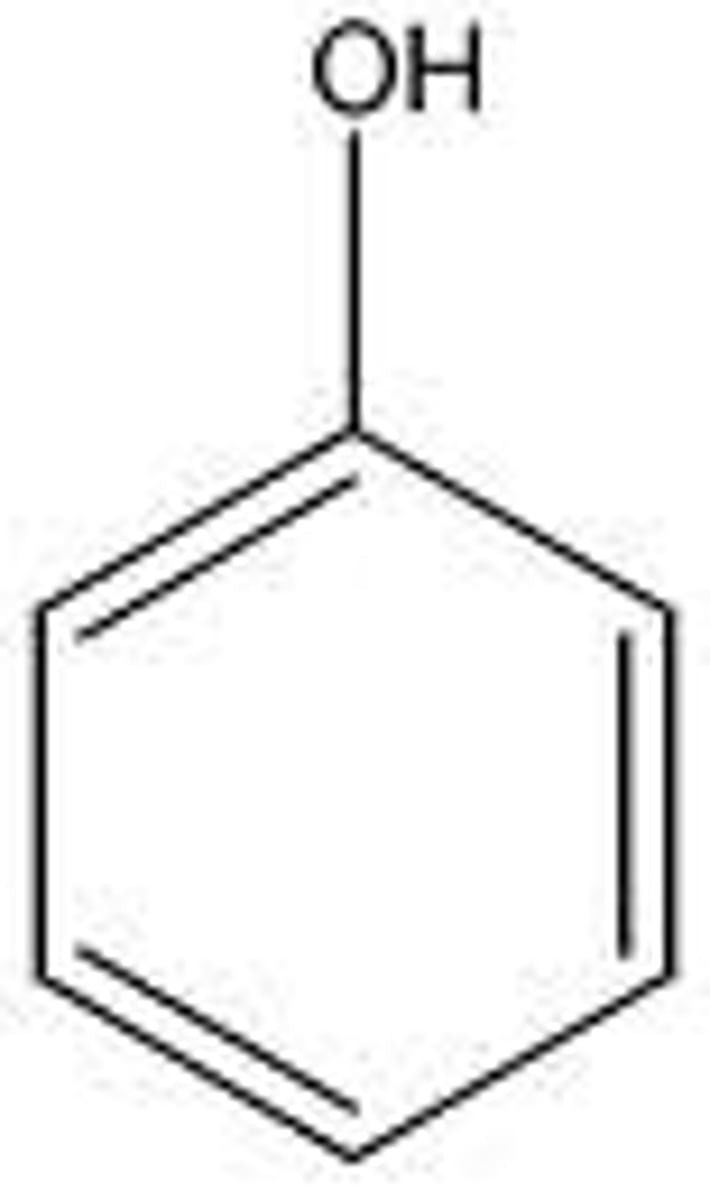
carboxylic acid
5

pKa table relation to acids and bases
More acidic molecules will have a smaller (or even negative) pKa. pKa below -2 are considered strong acids, which dissociate in aqueous solution
More basic molecule will have larger pKa.
endothermic
PE change from low to high
positive (+) or uphill
unfavorable
breaking a bond; energy must be added
exothermic
PE change from high to low
negative (-) or downhill
favorable
making a bond: energy be released by molecule
inductive effect
polarization caused by electronegative atom that can induce minor polarization in neighboring bonds
very weak
In one of the CTQ it talks about an H connect to an N or O is more acidic than H on C, F, or Cl. I am not sure tho.
Newman projections (lowest to highest PE)
remember lowest PE is always more favored
lowest: staggered
highest: eclipsed
staggered anti (180 degrees apart) > staggered gauche (60 degrees apart) > eclipsed (when same groups are right hidden by each other, the PE becomes even higher)
degree of unsaturation
no. pi bonds + no. rings
constitutional isomers
same molecular formula, different connectivity
conformers
identical
alternate way of showing the same molecule
stereoisomers
Compounds with the same structural formula but with a different arrangement of the atoms in space. (ex: one could be trans & other cis)
distereomers
stereoisomers that are not mirror images
2 types of orientation
Z (cis) = largest groups on same side of a double bond
E (trans) = largest groups on different sides of double bond
why is it favorable to put large groups in equatorial position?
- points away from the ring where there is more space
- lower PE conformation
isopropyl
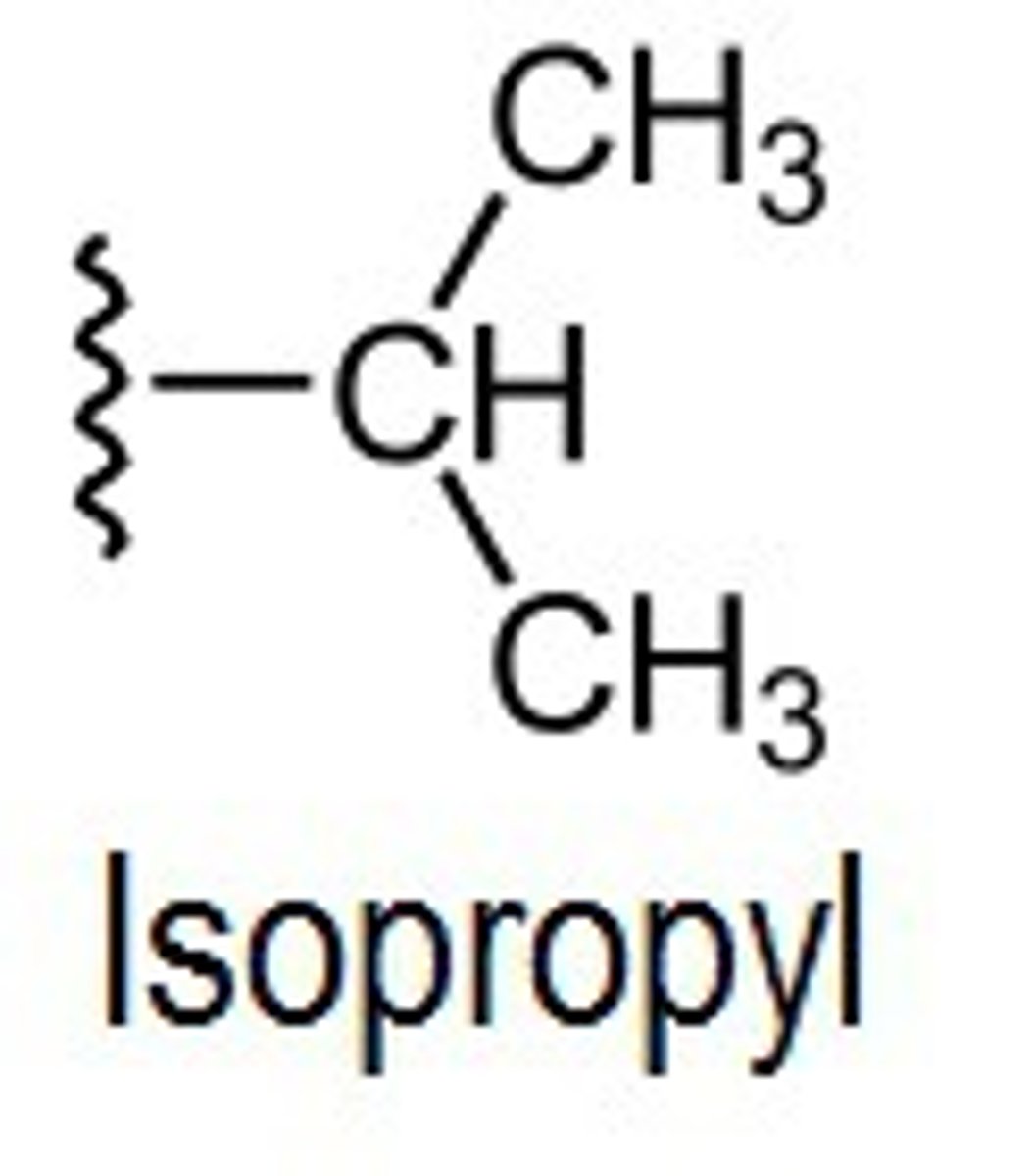
isobutyl

sec-butyl
CH3 - CH- [CH2CH3 (ethyl)]
![<p>CH3 - CH- [CH2CH3 (ethyl)]</p>](https://knowt-user-attachments.s3.amazonaws.com/6e521b1b-82ad-4d92-979d-0a5a6ceb3da8.jpg)
tert-butyl
Chapter 14 (& 15 a) memorization recap
Br2 hv => adds to most sub C
KOH heat => usually E2; since OH- is a small base, it obeys Zaitsev rule (double bond to most sub)
Large base E2 => Hoffman product
E1 reactions always follow Zaitsev's rule
NBS => Adds Br to most sub carbon. Next a small base or large base will follow. You add it according the whether it is an E2 or E1.
Chapter 15b recap
initiation = always breaks a bond to create 2 radicals
propagation step = usually 2 steps; and involves 2 breaks of excess bonds (H-Br, Br-Br, etc)
termination = combine leftover products
resonance structures acid & base
multiple resonance structures in acid = less acid - base = (-) downhill
multiple resonance structures in base = acid - less base = (+) uphill
M1 Q13