Science exam
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
Define ecosystems.
an ecosystem is an evironment where all the organisms interact with each other.
what is abiotic verses biotic?
abiotic is a non living thing and biotic is a living thing.
what is a producer verses a consumer?
a producer is something that creates its own food from sunlight.
a consumer is something that eats other organisms to gain energy.
what is the importance of a producer in ecosystem pyramids?
it is the starting point for the food chain where plants give their energy that they created to all the animals above.
what is photosynthesis?
it is when plants take solar energy to power themselves.
what is the role of a consumer in the ecosystem?
the role is transferring energy up the food chain. this can keep the population of lower animals in check since they have natural predators.
what is cellular respiration?
it is when plants produce glucose during photosynthesis and give the glucose to animals when they eat the plant.
what are Trophic levels and what is each level? give 2 examples each.
Trophic levels are feeding levels in the food chain.
Level 1 Producer: algae, and grass
level 2 Primary: bees, and grasshopper
level 3 Secondary: frog, and pigeon
Level 4 Tertiary: wolf, and dolphin
Level 5 Quaternary: shark, and polar bear
what are decomposers and their role in recycling nutrients?
they break down organic waste and bring it back into the earth.
why is sunlight the primary food source in an ecosystem?
plants need it to live and animals need plants to live so if there isn’t sunlight everything dies out.
what is niche? give 4 examples in an ecosystem.
when an organism has a specific job in the ecosystem.
ex) Giraffe which eats the leaves on acacia trees that other animals can’t eat.
ex) Dung beetle which consumes and buries animal feces that will put back the nutrients into the ground.
ex) Woodpecker which find insects and larvae beneath tree bark.
ex) hummingbird which feeds on nectar while hovering that will act as a key pollinator for other plants.
What is the difference between food chains and food webs? Give an example of each.
Food chain: a simple linear relationship that shows the energy goes through the ecosystem.
Food web: a diagram showing the eating habits of the ecosystem that consists of interlocking food chains.
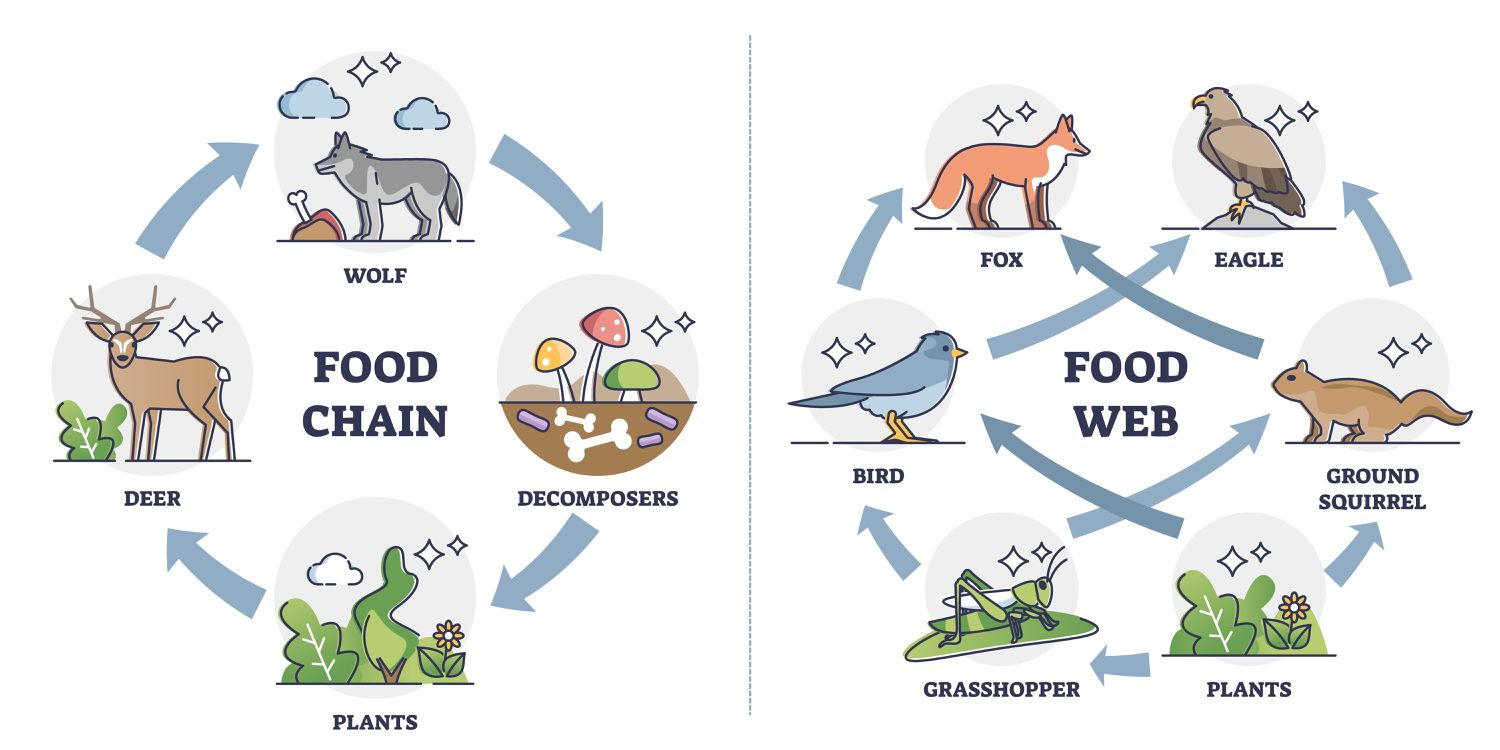
How does energy flow work?
lost as heat or waste. only 10 percent of animals energy flows to the next trophic level. this is called the 10 percent rule.
what are the 5 main steps in the Nitrogen Cycle? (does not need to be in order)
Nitrogen fixation
Ammonification
Nitrification
Assimilation
Denitrification
explain what each step in the Nitrogen cycle does.
Nitrogen fixation – Bacteria in some plants turn nitrogen gas into ammonia that plants can use.
Nitrification – Soil bacteria change ammonia into nitrites and then nitrates, which plants can absorb.
Assimilation – Plants take in nitrates to make proteins, and animals get them by eating plants.
Ammonification – Decomposers turn dead plants, animals, and waste into ammonia, which becomes ammonium in water.
Denitrification – Bacteria convert nitrogen compounds back into nitrogen gas when oxygen is low, releasing it into the atmosphere.
what are the 3 ways nitrogen fixation occurs?
Biological fixation: Bacteria in roots of Legumes and other bacteria do this conversion.
Atmospheric fixation: Lightning breaks nitrogen into nitrogen oxides, which are used in plants.
Industrial fixation: Ammonia is produced in factories to make fertilizers for farmers to grow better crops.
what are the names and chemical formulas of the following?
1. Nitrogen gas
2. Ammonia
3. Ammonium
4. Nitrate
5. Nitrite
Nitrogen gas: N2
Ammonia: NH3
Ammonium: NH4
Nitrate: NO3-
Nitrite: NO2-
What are 3 bacteria in the nitrogen cycle? describe their role and what they do.
Rhizobium
Converts nitrogen gas from the air into ammonia that plants can use.
Nitrosomonas
Changes toxic ammonia into nitrite through nitrification to prevent ammonia buildup.
Pseudomonas
Turns nitrates back into nitrogen gas, returning it to the atmosphere.
why is the nitrogen gas difficult to break down?
when there is a triple covalent bonds on two nitrogen atoms, it becomes almost impossible to spit apart.
why is the nitrogen cycle important for our ecosystem?
Plants and animals need nitrogen to make proteins. The nitrogen cycle turns nitrogen gas into usable forms with help from bacteria.
what methods are used to maintain nitrogen levels in the soil? (three methods)
Crop rotation
Different crops are grown in different seasons to balance soil nutrients, with legumes adding nitrogen to the soil.
Fertilizers
Fertilizers add nitrogen to the soil to improve plant growth when natural nutrients are low.
Summer fallow
Land is left unplanted for a season to restore water and nutrients for future crops.
what happens when there is too much nitrogen in an ecosystem?
Excess nitrates and ammonia make too many plants grow, harming lakes and rivers. They also cause algae and weeds to grow, which can release toxins that hurt fish and wildlife.
how do human activities affect the nitrogen cycle? (four ways)
Agricultural industry
Livestock produce manure that can release nitrates and ammonia into nearby water if not properly managed.Excessive fertilizer use
Fertilizers can wash into soil and water, contaminating groundwater and causing health problems.Septic fields and holding tanks
Leaks can allow sewage to seep into the ground and pollute drinking water.Water-treatment plants
Equipment failures or heavy rain can cause untreated sewage to enter rivers or lakes.
what is the carbon cycle and its role in the ecosystem?
the carbon cycle is when carbon atoms travel from the atmosphere to the earth and back, its role is to circulate carbon the building block of all life.
what happens during photosynthesis?
plants convert CO2 to and water into organic matter using light matter. the green pigment in plant cells are good to absorb light energy which is necessary for the process.
what is cellular respiration, and how does it contribute to the carbon cycle?
Organisms break down glucose to release energy for life processes. The carbon cycle moves carbon between the atmosphere and the Earth using respiration and photosynthesis.
how does decomposition return carbon to the ecosystem?
when something dies, decomposers break them down which releases carbon back into the soil and atmosphere.
what role does combustion play in the carbon cycle?
combustion of fossil fuels releases stored carbon into the atmosphere as CO2 which contributes to climate change.
what are the major carbon reserves in the environment? (four reserves)
Atmosphere: Carbon is in the air as CO₂. Plants use CO₂ in photosynthesis, starting food chains.
Oceans: Oceans absorb lots of CO₂, helping control atmospheric levels. CO₂ dissolves in water and enters aquatic food chains; respiration releases it back to the air.
Earth’s crust: Carbon is stored long-term in rocks and fossil fuels. Burning fossil fuels releases CO₂ and increases global warming.
Living organisms: Plants, animals, and decomposers cycle carbon by building tissues and releasing CO₂ through respiration.
how do organisms, the atmosphere, and oceans store carbon?
Organisms: moves through the animals when plants build tissue.
Atmosphere: Carbon is stored as CO2 in the atmosphere.
Oceans: absorbs the CO2 from the atmosphere as carbon.
How do human activity affect the carbon cycle?
Deforestation
Cutting down trees reduces the number of plants that absorb CO₂, lowering the ecosystem’s ability to regulate carbon.
what natural events release carbon into the atmosphere?
Forest fires
Forest fires destroy plants that absorb CO₂ and release large amounts of carbon into the atmosphere.
Volcanic eruptions
Volcanic eruptions release CO₂ and ash that blocks sunlight, reducing photosynthesis and increasing atmospheric carbon.
what is the oxygen cycle and why is it important?
The oxygen cycle is the circulation of oxygen atoms throughout the atmosphere, lithosphere, and biosphere. It is good for maintaining oxygen levels, which are essential for all life.
what is the role of photosynthesis in the oxygen cycle?
Photosynthesis gives energy to the plants and the plants inhale CO2 and exhale oxygen. this oxygen is important for the oxygen cycle so it can replenish.
how does cellular respiration use oxygen?
when an organism breathes, it takes in oxygen into its body. then it exhales CO2
What consumes oxygen and releases carbon dioxide?
cellular respiration
How are the carbon and oxygen cycles connected through photosynthesis and respiration?
Breathing takes in oxygen from the oxygen cycle and releases CO₂ into the carbon cycle.
Why are the oxygen cycle and carbon cycle important to maintain balance in the ecosystem?
Oxygen cycle
The oxygen cycle moves oxygen around the Earth, which is essential for animals to breathe.
Carbon cycle
The carbon cycle moves CO₂ between the atmosphere, water, and living things.
what are greenhouse gases, and how does it affect the earths atmosphere?
it is CO2 in the atmosphere which creates a blanket over the atmosphere. this can trap heat inside and heat up the planet. when there are more greenhouse gases, the suns heat enters the atmosphere but the excess heat can’t escape into space. this is what the main contributor to global warming is.
what is population density?
the amount of people per square unit.
what is biotic potential?
the amount of offspring a species can produce if resources were unlimited.
what are the 4 factors that affect biotic potential?
Birth potential: the maximum number of offspring an organism can produce per birth
Capacity for survival: the number of offspring that reach reproductive age.
Procreation: the number of times a species can reproduce each year.
Length of reproductive life: the number of years an individual can reproduce.
what are the three types of survivorship curves with examples?
Type 1: high survival rate in early and middle life.
ex) humans, bears
Type 2: high mortality rate across life time.
ex) birds, squirrels
Type 3: high mortality rate in early life but survivors live long.
ex) fish, insects
what are survivorship curves?
shows how likely an individual is going to reach a certain age.
how does reducing a population impact other organisms?
one population decreases, the animals that rely on it as their main food source may also decline because there’s less food available for them.
what is trophic cascade?
when a predator is taken or added to the food chain it can make direct or indirect changes.
2 examples of direct and indirect disruptions from trophic cascade.
Direct examples: Wolves eat elk, reducing their population. Sea otters eat sea urchins, controlling their numbers.
Indirect examples: Without wolves, elk overgraze, lowering aspen and willow populations. Without sea otters, sea urchins overeat kelp, destroying kelp forests.
what is carrying capacity?
the maximum number of organisms from a species that an ecosystem can support indefinitely.
what happens if a population exceeds its carrying capacity?
the death rate can increase
reproduction can decrease
population size will decline
once the population goes below the carrying capacity, it will start to increase and stabilize due there being enough resources for the species to survive and thrive.
how does carrying capacity and energy pyramid work together?
the amount of energy at the producers level determines the population size the ecosystem can sustainably support at higher trophic levels.
what is density dependent? 3 factors
when the effects increase as the population grows.
competition: as resources become scarce, competition for food, water, and space intensifies.
predation: larger prey populations can attract more predators to the area, which lowers the prey population.
disease: close contact in high density areas can spread disease more easily.
what is density independent? 2 factors
when the effects are just as bad no matter how large the population is.
natural disasters: events like floods, wildfires, or droughts can reduce the population, no matter the size.
human activities: pollution, habitat destruction, and deforestation are human changes that can reduce the population of habitat.
give an example each of density dependent and density independent factors.
Density-dependent: a disease outbreak among deer can spread rapidly if the population is large, leading to a sudden drop in numbers.
Density-independent: a forest fire might wipe out part of a population regardless of whether it was large or small.
what is bioaccumulation?
the gradual buildup of chemicals in an organism over time.
what is biomagnification?
the buildup of substances by successive trophic levels.
how do toxins and DDT build up at higher trophic levels?
predators eat contaminated prey, so all the toxins in the prey build up and get concentrated in the predators body.
what are the effects of bioaccumulation on organisms at higher trophic levels?
it can affect growth, reproduction, immune, and development. can also cause liver and nervous system damage. linked to birth defects and developmental delays in infants.
what is a non-native invasive species?
when a species negatively affects a habitat and biodiversity.
what is the difference between a native species and non-native invasive species?
Non-native invasive species
A species from another area that harms the local environment.
Native species
A species that naturally lives in an area with predators and prey that control its population.
what are 4 traits that enable invasiveness?
have few natural predators, competitors, parasites, or diseases.
are long lived.
have high reproduction rates.
can feed on a wide variety of things and thrive in many environments.
what is an example of an invasive species in Manitoba?
European buckthorn
how does European buckthorn disrupt native populations and ecosystem balance?
It is a very dense plant that requires a lot of nutrients, so it shades out all the native plants around it and takes all of its nutrients to live, which kills off the native plants.
what are 3 techniques to manage European buckthorn from growing?
putting chemicals on the bark or leaves to kill it.
natural predator eats it
burning or pulling the plant out of its roots.
what is an atom?
it is the fundamental building block of matter and is the smallest chemical with elements properties.
what are the 3 subatomic particles? include their charge and location in the atom.
Proton: positively charged in the nucleus.
Electron: negatively charged orbiting the nucleus.
Neutron: neutral charged in the nucleu
what is the atomic number of an element?
the number of protons in the nucleus of an element.
how does the atomic number determine which element an atom is?
it represents the number of protons in the nucleus of the atom.
how is atomic mass calculated?
it is the weighted average of the neutrons and protons.
what is an electron shell and what is their importance in chemical bonding?
an electron shell is the orbits around the nucleus. atoms want a full valence shell because it makes them stable.
how many electrons can the first and second shells hold?
the first one holds 2 electrons and the second one holds 8 electrons.
what is the periodic table?
a chart that organizes elements to their properties and how they behave in chemical reactions.
How is the periodic table organized by atomic number, groups, and periods?
Atomic number: numbers increase as you go left to right and top to bottom.
Groups: elements in the same group act similarly because they have the same number of outer electrons.
periods: all elements in the same row have the same amount of electron shells.
list properties of alkali metals and examples. (group1)
highly reactive and easily lose their one valence electron.
soft metals with low melting points.
reacts strongly with water to create hydrogen gas.
ex) lithium, sodium, potassium
list properties of alkaline earth metals and examples. (group 2)
have 2 valence electrons and are less reactive than alkali metals.
form alkaline solutions when mixed with water.
ex) beryllium, magnesium, calcium
what are chalcogens?
a group of elements in the oxygen family. group 16.
list properties of chalcogens and examples.
referred to as oxygen group.
have 6 valence electrons.
form compounds with oxygen and sulfur.
ex) oxygen, sulfur, selenium
what are halogens and what are diatomic molecules for them?
group 17 of the periodic table. all natural halogens form diatomic molecules, meaning each molecule is made of 2 identical atoms bonded together.
List properties of halogens and examples.
highly reactive non-metals that require one more electron to get a full valence shell.
exist as diatomic molecule as a natural state.
ex) fluorine, chlorine, bromine
what are noble gases and their stability?
Group 18 elements have full outer electron shells, making them stable. They don’t need to gain, lose, or share electrons, so they are unreactive.
list properties of noble gases and examples.
known for their stability due to having a full valence shells of electrons.
extremely unreactive and rarely form compounds.
used in lighting such as neon lights.
ex) helium, neon, argon
whats an ionic bond?
when the electron are transferred from one atom to another, creating ions with opposite charges.
how do metals and non-metals form ions?
when lots of opposite charges are attracted to each other it creates ions which occurs usually between metals and non-metals.
what are the steps to combining a metal and a non-metal to form an ionic compound?
identify which is the metal and which is the non-metal.
determine the charges
transfer the electrons - Metal loses electrons, non-metal gains electrons.
Opposite charges attract → ions stick together.
Balance charges so the total positive and negative charges cancel out.
Write the formula (metal first, then non-metal).
What are the physical properties of ionic compounds?
High melting and boiling points
Hard and brittle
Do not conduct electricity as solids
Conduct electricity when melted or dissolved in water
Form crystal lattice structures
Write the formula for the compounds formed between magnesium and chlorine.
MgCl₂
Mg → Mg²⁺
Cl → Cl⁻
Two Cl⁻ ions are needed to balance one Mg²⁺
Write the formula for the compounds formed between calcium and oxygen.
CaO
Ca → Ca²⁺
O → O²⁻
Charges balance in a 1:1 ratio
Write the formula for the compounds formed between aluminum and sulfur.
Al₂S₃
Al → Al³⁺
S → S²⁻
Balanced ratio: 2 Al for 3 S
What is a covalent bond?
Covalent bonds happen when atoms share electrons evenly. They usually form between non-metals and make up molecular compounds.
What are diatomic molecules?
A diatomic molecule is made up of two atoms of the same non-metal element sharing a pair or pairs of electrons.
What are the 7 diatomic elements?
Hydrogen, oxygen, flourine, bromine, iodine, nitrogen, chlorine
H, 0, F, Br, I, N, Cl
What are the physical properties of covalent compounds?
Low melting and boiling points
Usually soft or brittle
Do not conduct electricity
Can be gases, liquids, or solids at room temperature
Often soluble in non-polar solvents (some polar ones dissolve in water)
What is the covalent compound formula for two nitrogen atoms and five oxygen atoms?
N₂O₅
2 Nitrogen (N) + 5 Oxygen (O) → N₂O₅
What is the covalent compound formula for one phosphorus atom and three bromine atoms?
PBr₃
Phosphorus (P) + 3 Bromine (Br) → PBr₃
What does a Lewis Dot Diagram show?
Highlights the most important electrons in the outermost shell.
Write the steps for naming ionic compounds?
Identify the ions (a metal cation and a nonmetal or polyatomic anion).
Write each ion with its charge.
Balance the charges so the total charge is zero.
Adjust subscripts to balance charges (do not change charges).
Write the final formula and remove charges.
Name: What is MgO?
Magnesium oxide
Name: What is FeCl3?
iron trichloride
Write the steps for naming covalent compounds?
Memorize numeric prefixes for naming covalent compounds.
Use prefixes from the formula to indicate the number of atoms.
Add prefixes before the element names.
Change the ending of the second element to "-ide".
What are these covalent compound: P2O5, SF6, and N2O4
diphosphorus pentoxide, sulfur hexafluoride, dinitride tetraoxide
name the first 20 elements in the periodic table.
Hydrogen (H)
Helium (He)
Lithium (Li)
Beryllium (Be)
Boron (B)
Carbon (C)
Nitrogen (N)
Oxygen (O)
Fluorine (F)
Neon (Ne)
Sodium (Na)
Magnesium (Mg)
Aluminum (Al)
Silicon (Si)
Phosphorus (P)
Sulfur (S)
Chlorine (Cl)
Argon (Ar)
Potassium (K)
Calcium (Ca)