chem 107 4/3/25 7.4
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Fats Are Solids; Oils are Liquids
Fats, including steak, lard and butter are derived from
animals.
• Oils, like those from corn, soybeans, and peanuts are
derived from plants.
• Both fats and oils belong to a class of hydrolyzable lipids
called triglyercerides.
• Determining a fat versus an oil is as simple as examining
the physical state of the triglycerides at room
temperature – oils are liquids and fats are solids
Fatty Acids
A carboxylic acid with a very long hydrocarbon chain!
1. Saturated fatty acids1. Saturated fatty acids
– long van der Waal zippers!!

Conformational Isomers
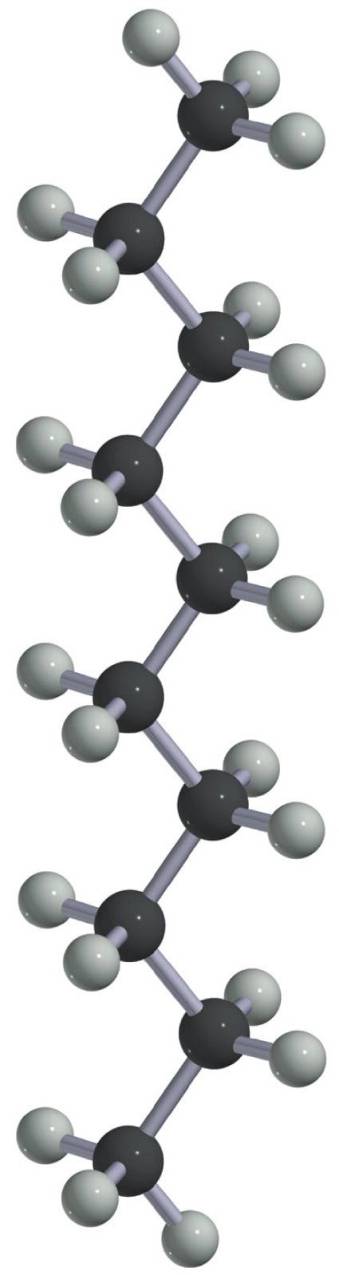

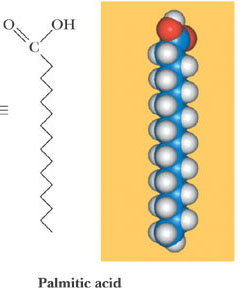
Fatty Acids
Naturally occurring fatty acids
• Usually have an even number of carbon atoms
• Usually are unbranched
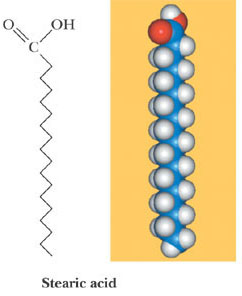

Fatty Acids
A carboxylic acid with a very long hydrocarbon chain!
1. Saturated fatty acids1. Saturated fatty acids
– long van der Waal zippers!!long van der Waal zippers!!
2. Unsaturated fatty acids2. Unsaturated fatty acids

Unsaturated Acids
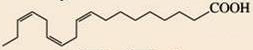
Unsaturated Hydrocarbons
Intermolecular interaction = van der Waal forces

Unsaturated Hydrocarbons

Unsaturated Hydrocarbons

Fatty Acids
. Naturally occurring fatty acids
• Usually have an even number of carbon atoms
• Usually are unbranched
• Unsaturation isomers are cis C=C

Dietary Lipids
The oil has many unsaturated cis carbon-carbon double
bonds in the hydrocarbon tail.

Dietary Lipids
In the ball-and-stick model of the fat, notice that the tails
are physically close to each other.
• This proximity creates disturbances in the electron
distribution around each of the tails as they interact with
each other.
• These disturbances result in London forces – the tails of
the fat are attracted to each other.
• These mostly straight-chain tails allow for many surface
contacts, which increases the attractions between the tails
and restricts the molecular motion, allowing the molecules
to form a solid or a semisolid.
Dietary Lipids
Look at the ball-and-stick model of the oil molecule.
• It has many unsaturated cis carbon-carbon double bonds in the hydrocarbon
tails, making it polyunsaturated.
• Notice how the cis double bonds in the tails of the oil creates kinks in the
otherwise straight hydrocarbon chain.
• The kinked tails interact less with each other through London forces because
the tails of the unsaturated oil cannot stack together as closely as those in the
fat can.
• Less London force attractions means that the hydrocarbon chains in the oil
move more freely.
• The greater molecular motion among the hydrocarbon tails in an oil does not
allow enough stacking of the tails for a solid to form at room temperature.
Attractive Forces and the Cell
Attractive Forces and the Cell Membrane
A Look at Phospholipids
• The main structural components of cell membranes are called
phospholipids.
• Phospholipids have a glycerol backbone with fatty acids linked
to it through an ester bond.
• Phospholipids have only two fatty acids on their glycerol
backbone. The third OH group of the glycerol is bonded to a
phosphate-containing group.
• The phosphate-containing group is ionic (polar).
• The fatty-acid tails are nonpolar.
Attractive Forces and the Cell
Membrane (2 of 9)

A Look at Phospholipids
There are many different phospholipids, but they all share
this similar structure: a glycerol backbone with two nonpolar
fatty acid tails and a polar phosphate-containing head.
• The two tails of the phospholipid affect the overall shape of
the molecule.
• Phospholipids have a cylindrical shape with a much
larger head group, which hinders their ability to form
micelles.
The Cell Membrane Is a Bilayer
A cell membrane composed of phospholipids cannot exist as
a single layer.
• The phospholipid bilayer is the structural foundation for a
cell’s membrane.
• Instead, the phospholipids form a double layer called a
bilayer.
• The polar heads are directed out into the surrounding aqueous
environment and into the aqueous interior of the cell.
• This arrangement leaves the hydrophobic tails of both layers
directed toward each other, creating a nonpolar interior region.
Attractive Forces and the Cell
Attractive Forces and the cell Membrane (5 of 9)

Attractive Forces and the Cell Membrane (6 of 9)
Protein molecules can span the bilayer (integral membrane
proteins) or associate with one hydrophilic surface of the bilayer
(peripheral membrane proteins).
• Proteins are the membrane’s functional components, allowing
selected molecules to move into and out of the cell.
• The exterior surface of the cell membrane also contains
carbohydrates that act as cell signals.
• The fluid mosaic model creates “icebergs” of protein floating in
a “sea” of lipids.
• The membrane is fluidlike: The phospholipids move freely within
their bilayer.
7.5 Attractive Forces and the Cell Membrane (7 of 9)

Attractive Forces and the Cell Membrane
(8 of 9)
Steroids in Membranes: Cholesterol
Cholesterol is a steroid.
• Steroids are lipids with a structure that
contains a four-membered fused ring called
a steroid nucleus.
• Even though the steroid structure differs
greatly from the structures of fatty acids
and triglycerides, steroids are classified
as lipids based on their nonpolar
nature.
• These molecules have a variety of
functions in the body, including regulating
sexual development (testosterone and
estrogens) and emulsifying dietary fats
(bile acids).

7.5 Attractive Forces and the Cell
Membrane (9 of 9)
Steroids in Membranes: Cholesterol
• Cholesterol contains the steroid nucleus with a polar end.
• Cholesterol situates itself in the phospholipid bilayer so that the -OH
group protrudes out into the aqueous
environment, while the rest of the
molecule nestles in the nonpolar
interior of the membrane.
• Cholesterol can slip in between the
saturated hydrocarbon tails of
phospholipids, increasing fluidity.
• Cholesterol can also interact with
unsaturated tails, increasing the
rigidity of the membrane.