CHM1022 – Organic and Inorganic Chemistry Exam Revision Questions
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
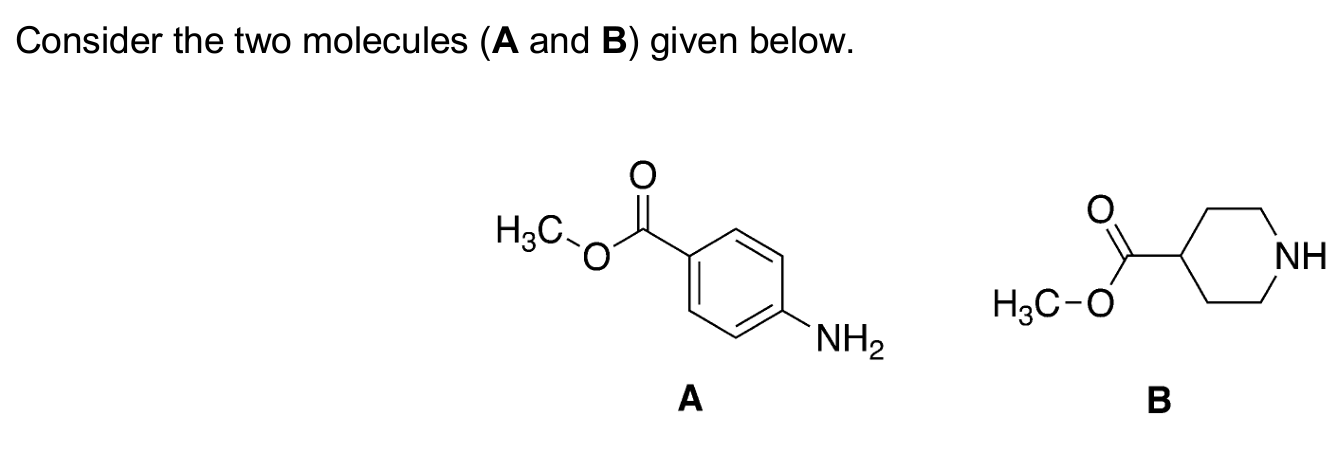
Identify which molecule is a weaker base
In molecule A nitrogen lone pair is withdrawn from aromatic ring by ester functional group (which decreases the likelihood of nitrogen accepting protons, which is what a base does)
In molecule B there is no conjugation (no overlap of pi-orbitals) and therefore the nitrogen lone pair is able to donate protons (= base)
Resonance stabilisation increases stability of a molecule. If asked “which is weaker base” and since bases accept H+ which requires lone pair of electrons, resonance structures would reduce the strength of the base since the electron is not readily available on the N atom to accept H+.
What determines if a molecule is chiral
Contains one or more stereogenic centres
Is not superimposable on its mirror image (i.e., enantiomer)
Why is an alkene nucleophillic
Contains a C=C double bond, which can donate a pair of electrons to an electrophile to form a covalent bond
What is the condensed electron configuration of Ag+ and Pd4+
[Kr]4d10 (1 mark)
[Kr]4d6 (1 mark)
Name an example of a neutral bidentate ligand that contains nitrogen
Bipyridine (bpy), ethylenediamine (en), ethanolamine
(oxalate is also a bidentate ligand, but it doesn’t contain nitrogen– contains 2- charge)
List all the donor atoms in [Mn(Br)(en)(SCN)(OH2)]+.
Br, N, S, O
Name one example of a polydentate ligand (with a denticity of 3 or greater)
EDTA4- (hexadentate ligand, 4 carboxylate oxygen + 2 amine nitrogen), diethylenetriamine (dien), porphyrin (tetradentate ligand, macrocycle, 4 nitrogen donors)
Consider an aqueous solution of Fe(NO3)2 when it is combined with a small amount of ammonium and ethylenediamine. Name two potential ligands present in this solution. (2 marks)
Nitrate, aqua, ammine, ethylenediamine
b. What complex would you expect to form predominantly in this solution? Justify your response. (3 marks)
[Fe(en)3]2+ or [Fe(en)3](NO2)2 or triethylenediamineiron(II) (1 mark) Ethylenediamine is a bidentate ligand. (1 mark) It is most thermodynamically stable – as denticity increases, thermodynamic stability increases. (1 mark)
a. In general, which modifications of an octahedral transition metal complex would increase the size of ∆oct? (3 marks)
Using stronger field ligands e.g. NH3 instead of H2O (1 mark)
Increasing oxidation state of the central metal e.g. Cr(III) instead of Cr(II) (1 mark)
Replacing a first row transition metal centre with a second row transition metal centre e.g. Mo instead of Cr (1 mark)
b. In a tetrahedral splitting pattern, describe which orbitals are: (2 marks)
i. Higher in energy ii. Lower in energy
dxy, dxz, dyz (1 mark)
dx2-y2 and dz2 (1 mark)
a. For the following pairs of complexes identify which complex will have a larger ∆oct. (2 marks)
i. [Fe(CN)6]4- vs [Fe(NH3)6]2+
[Fe(CN)6]4- (1 mark)
ii. [Cr(NH3)6]3+ vs [Cr(NH3)6]2+
[Cr(NH3)6]3+ (1 mark)
b. Three different aqueous solutions contain one of the following ions (where M is a transition metal ion):
1. [M(en)3]3+
2. [MF6]3–
3. [M(ox)3]3–
Use the spectrochemical series and the colour wheel provided to predict which of the following observed colours - blue, violet and yellow - correspond to the three metal complexes provided.
1. [M(en)3]3+ Yellow
2. [MF6]3- Blue
3. [M(ox)3]3- Violet
a. Haemoglobin is used to transport oxygen around the human body.
Describe the haem metal complex, referring to the metal centre and the ligand system in its deoxy form, and how this subunit is connected to the rest of the haemoglobin species. (4 marks)

b. Provide two reasons why small molecules such as carbon monoxide may be toxic to humans. (1 mark)
CO is very good at binding to haem (0.5 marks) Both molecules bind 200 times stronger than O2 (0.5 marks)
Which of the following statements is true of strong field ligands such as CN/CO/NH3?
They usually produce low spin complexes (due to increased electron-electron repulsion when spin-paired) and high crystal field splittings
Which of the following statements is true of weak field ligands such as I/Br?
They usually produce high spin complexes and low crystal field splittings

Which one of the following coordination complexes would you expect to be the most stable (i.e. has the highest formation constant)? Select one:
[Co(EDTA)]- because it’s a polydentate ligand (i.e., hexadentate ligand, a chelating ligand) which involves coordinating to a metal ion using more than one donor atom, to stabilise the coordination complex

Identify the donor atoms present
Cl, O, N (I, II and III)
en = ethylenediamine (which contains 2 nitrogen atoms that are the donor atoms within the bidentate ligand)
If the oxygenated form of haemoglobin results in red blood, what colour and wavelength does it absorb in?
Blue/green (490 nm)
What is the correct description of d electrons in a paramagnetic transition metal complex? Select one:
a. Only observed for TM complexes with a 'high-spin' configuration.
b. Only observed for TM complexes with zero unpaired d electrons.
c. Only observed for TM complexes with one or more unpaired d electrons.
d. Only observed for TM complexes with two or more unpaired d electrons.
Paramagnetic properties of transition metal complexes are only observed for transition metals with one or more unpaired d electrons
What is the correct description of d electrons in a diamagnetic transition metal complex?
Diamagnetic properties of transition metals are only present for transition metals with zero unpaired electrons (i.e., high spin and strong field ligands)

What is the condensed electron configuation?
Ignore the 5s orbital, fill in the 4d orbitals first

What is the correct order for increasing chemical shift?
I, III, II
Transition metal complexes often exhibit colour. True or False: The colour corresponds to the wavelength of visible light emitted by the compound.
False.
The colour of transition metals is due to the wavelength of visible light that is absorbed. The colour observed is due to the wavelength of visible light that is not absorbed (i.e., the remaining light transmitted/reflected)
Carbonic anhydrase is an enzyme found in red blood cells. What metal is responsible for its catalytic activity?
Cu2+, Zn2+
The correct name for the compound, [Co(NH3)5 (NO2)]Cl2, is
pentaamminenitro-kN-cobalt(III) chloride

What compound has the coordination number of 6?
[CoCl2(en)2]2+
What does conjugation mean?
Refers to a system where alternating single and double/triple bonds allow for the delocalisation of adjacent p-orbitals
Enables for a more stable electronic structure since the electrons in the pi bonds are not conbined to the space between atoms, but spread over multiple atoms = resonance structure
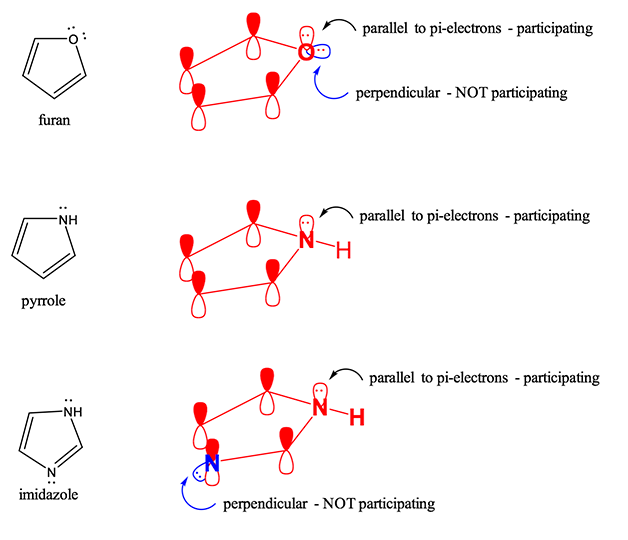
Is furan an aromatic?
Yes, because while the oxygen has 2 lone pairs, one is parallel with the p-orbitals thus participates in the pi-system, HOWEVER one is perpendicular with the p-orbitals thus does NOT participate in the pi-system and is not considered a pi-electron
Altogether = 2 + 2 + 2 = 6 pi-electrons
What is the spin-only magnetic moment formula?
√n(n+2) B.M.
where n = number of UNPAIRED electrons
What is the crystal field theory
Describes the bonding between the metal ion and ligand being largely electrostatic, and that the strength of the metal ion to ligand bond affects splitting of energy levels of d-orbitals
What is the relationship between atomic radius and effective nuclear charge?
As you move across the periodic table (LHS → RHS) the atomic radius decreases bc as you move along, the effective nuclear charge (Zeff) increases.
The number of core electrons remains constant, whereas the number of protons increases. This means that pull from the nucleus, experienced on the valence electrons, is greater as you move across. Thus atomic radius decreases.
While this is present for the transition metals, atomic radius decreases slightly (stays relatively constant). This bc the filled inner d-orbitals help to shield the outer 4s orbitals from the effective nuclear charge (Zeff).
Lewis acid
Accepts electrons from a donor (i.e., carbocation, electrophiles)
(different from Brønsted-Lowry theory, which involves donating protons)
Lewis base
Donates electrons (i.e., H-Cl, nucleophiles)
(different from Brønsted-Lowry theory, which involves accepting protons)
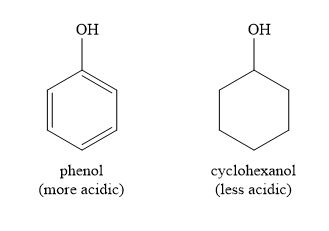
Why is phenol a stronger acid than cyclohexanol?
Phenol is a strong acid than cyclohexanol bc it donates a proton to become a phenoxide ion, the negative charge on the oxygen atom can be stabilised through resonance. This is possible because phenoxide is aromatic and has a conjugated pi-system.
This is not possible for cyclohexanol bc it is not aromatic or conjugated = no possible resonance structures available
Examples of ambidentate ligands (i.e., produce linkage isomers)
CN-, SCN- and NO2-
Coordinate with the metal ion through two different donor atoms
Examples of monodentate ligands
H2O, NH3, CN, Cl-, pyrrole
Examples of bidentate ligands
ethlyenediamine (en), C2O42- (oxalate)
Examples of polydentate (multidentate) ligands
diethlyenetriamine (dien), ethlyenediaminetetra acetic acid (EDTA4-) → hexadentate ligand (4 carboxylate oxygen + 2 amine nitrogen donor atoms)
4s-3d Filling Exception
3d orbitals are lower than 4s for Sc→Zn (except K/Ca) so electrons populate d-orbitals (n) before s-orbitals (n-1) (i.e., fill 3d before 4s, fill 4d before 5s = aufbau’s principle is not always obeyed for TM)
D-orbitals are not degenerate (i.e., d-orbitals do not share the same energy levels)
Can polypeptides serve as ligands for TM?
Yes, bc some amino acid side-chain may contain S, N or O atoms (aka. heteroatoms)
What geometry does cis-platin have?
Square planar
Explain the process of oxygen binding to haemocyanin
Deoxyhaemocyanin: Cu+, active site is deoxygenated and the 2 Cu atoms are far apart
Oxyhaemocyanin: Cu2+ (binuclear Cu2+ centre), active site is oxygenated and as O2(g) binds the Cu centres are bridged together
What is the requirement to form ligands with TM?
The amino acid side chain must contain heteroatoms (N, S or O)
How does the platinum anti-cancer drug, cis-platin, function?
The two H2O ligands on platinum are replaced by adjacent guanine bases = DNA strand unwinds = inhibits further replication and transcription of DNA.
Initially, in cancerous cells, [Cl-] = low thus cis-platin exchanges chloride ligands with H2O (0→1→2) to form a charged complex
Zinc-Based Enzymes
Does not change oxidation state (generally redox stable)
Usually found coordinated with histidine/cystiene amino acids (3 via N atoms and 1 H2O → tetrahedral arrangement)
Found predominantly in RBCs and responsible for the catalysis of reversible hydration of CO2(g) to form the bicarbonate ion and proton

What is the correct order of increasing chemical shift?
I, III, II
The H atoms around the alkyl are shielded, near the oxygen are shielded and on the C=C double bond are deshielded bc it induces its own magnetic field
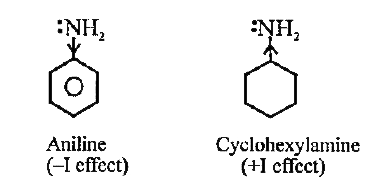
Why is aniline a weaker base compared to cyclohexylamine?
Base: accepts H+, requires electrons
Acid: donates H+, does NOT require electrons
Aniline is a weaker base because the lone pair on nitrogen is delocalised within the benzene ring, specifically it interacts with pi-electrons through resonance thus it has a reduced ability to accept H+. But… this means aniline is a stronger acid (favourable without electrons) since the N-H2 bond is weaker. The conjugate base is stabilised through resonance, which means its derived acid = strong.
Cyclohexylamine is not conjugated, the lone pairs on nitrogen are still there so it can readily accept H+ = base.
Why is this molecule chiral
YIt contains one or more stereogenic centres (1 mark) It is not superimposable on its mirror image or enantiomer (1 mark)
If the compound is blue would this have weak field or strong field ligands?
Weak field ligands (LHS of spectrochemical series) since they adopt a high spin state (due to large number of unpaired electrons) and thus absorb longer wavelengths of light
They will absorb orange wavelengths of light and thus transmit (or reflect) blue wavelengths (according to the colour wheel)
If you transmit/reflect blue colours (short wavelength) that means you absorbed red-orange (long wavelength) colours.
Short wavelength = small ∆oct = high spin state = absorb long wavelengths
Long wavelength = large ∆oct = low spin state = absorb short wavelengths
Discuss the similarities between iron and zinc

Discuss the differences between haemoglobin and haemocyanin

![<p>Why are there 2 geometric isomers for [CoCl2(en)2]+ but only 1 isomer for [CoCl2(en)]-?</p>](https://knowt-user-attachments.s3.amazonaws.com/2889d32e-2b66-4a90-835c-65a8fdae12e9.png)
Why are there 2 geometric isomers for [CoCl2(en)2]+ but only 1 isomer for [CoCl2(en)]-?
Ethylenediamine can only coordinate to two cis positions and therefore cannot form a trans isomer for [CoCl4(en)]–. With only two different ligands, at least two bidentate ligands are required around an octahedral metal centre for the complex to be chiral. Chiral complexes could also be obtained with bidentate ligands replaced by pairs of new monodentate ligands (e.g. [CoCl2(OH2)2(en)]–, but this is not the case here.
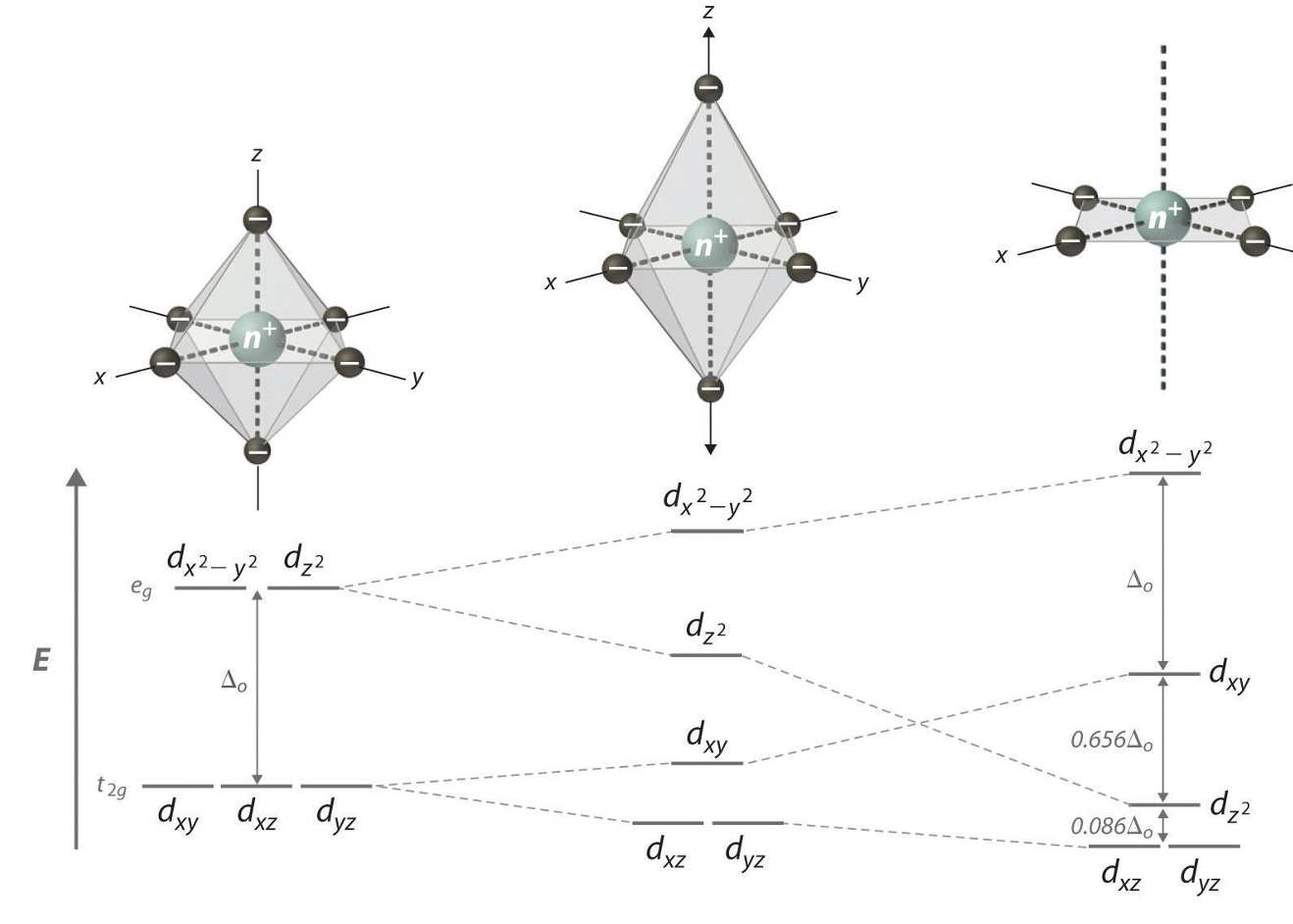
Square planar pattern
What is the difference between first ionisation energy and effective nuclear charge
First ionisation energy refers to the energy required to remove the first valence electron from the valence shell.
Effective nuclear charge referee to the electrostatic attraction between the positive nucleus and the negative electrons within an atom.
What happens to the first ionisation every as you go down group 6 elements?
First ionisation increases, rather than the general trend of decreasing. This is because atomic radii of the transition metals in general stays relatively the same (as you go down the group) due to lanthanides contractions (which is just a steady decreasing in the size of atoms for rare earth elements).
Because of this, the effective nuclear charge increases, whilst the atomic radii stays relatively similar. Therefore there is an increase in the amount of energy required to remove the first valence electron.
Explain why the atomic radii of molybdenum and tungsten at the same
This is due to the presence of lanthanides contractions, where the 4f electrons poorly shield the 5d electrons in transition metals causing the atomic radii to change minimally
True or False: as oxidisation state increases, so too does the tendency to remove electron decrease, this makes chromium more electronegative and more acidic.
Tendency to donate electrons decreases, means that there is a greater likelihood of gaining electrons. Which is donating proton = characteristic of acid (more electronegative)
The relative energies of 3d and 4s fro a gas phase or makes atom/ion
Note that when removing electrons we always take it from the 4s orbitals, because they are always lost from the highest energy level. 4s orbitals have a lower energy than 3d so they are filled first.
The relative energies of 3d and 4s for a coordination complex or dressed atom/ion