6.7 Nucleophiles and Electrophiles
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What are ionic (polar) reactions?
One molecule has extra electrons, and the other needs electrons
The electron rich part is attracted to the electron poor part
How common are ionic reactions in organic chemistry?
They make up about 95% of all organic reactions
Why do ionic reactions happen?
Because opposites attract- the electron rich part of one molecule reacts with the electron-poor part of another
What are the other types of reactions besides ionic?
Radical reactions and pericyclic reactions- these are less common and studied later in the course
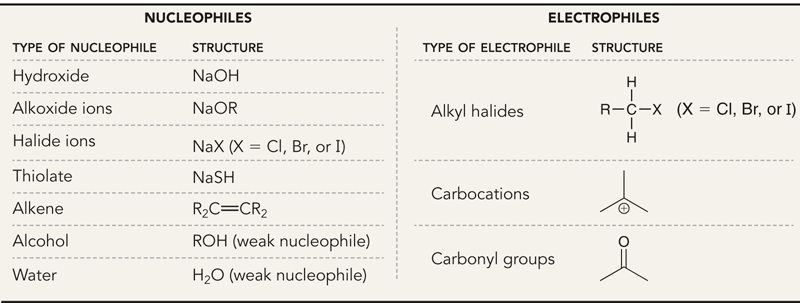
What is a nucleophile?
Electron rich and donates a pair of electrons to something positive
It is called nucleus lover because it is attracted to positive charges
What is an electrophile?
Electron-poor and can accept pairs of electrons
Called electron lover because it is attracted to negative charges
What is the relationship between nucleophiles and Lewis bases?
Nucleophiles are Lewis bases because they both donate electrons
What is the relationship between electrophiles and Lewis bases?
Electrophiles are Lewis acids because they both accept electrons
How do nucelophiles and electrophiles interact?
The nucleophile gives electrons to the electrophile, forming a new bond
What is a strong nucleophile?
Usually has a negative charge (anions) and is ready to share with something positive
Ex: HO-, RO-
What is a weak nucleophile?
Has no negative charge but has a lone pair
Examples: H2O (water) & R-OH (alcohols)
What is polarizability?
It is how easily an atom’s electron cloud can move or stretch when near something positive
How does polarizability affect nucleophilicity?
The more polarizable an atom is, the stronger a nucleophile it becomes because its electrons can reach and attack easier
Which atoms are very polarizable and strong nucleophiles?
I-, HS-, Br-, and Cl- they are big atoms with loose, easily moved electrons
Why can alkenes act as nucleophiles?
Because their π bonds have electrons that can attack positive atoms (electrophiles)
Do alkenes have a negative charge?
No, but their π electrons are exposed and can still act like a nucleophile in reactions
Examples of alkenes

What is an electrophile?
Electron-poor atom or molecule that can accept a pair of electrons
Electrophiles are Lewis acids
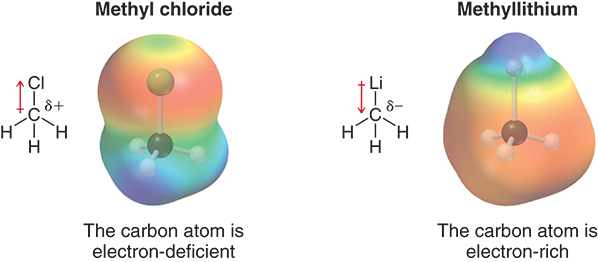
Why are alkyl halides electrophilic?
Because the halogen pulls electrons away from the carbon, making the carbon slightly (δ⁺) and electron-deficient
What is a carbocation?
A carbon with a full positive charge (+) and an empty p orbital
Very electron-poor, so it is a strong electrophile
What do electrophiles and nucleophiles do in a reaction?
The nucleophile donates electrons to the electrophile, forming a new bond
What is a carbonyl group?
A double bond between carbon and oxygen (C=O)
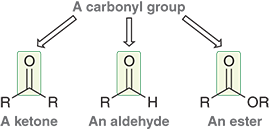
Why is the carbon in a carbonyl group electrophilic?
Oxygen pulls electrons away, making the carbon partially positive (δ⁺) and electron poor
Summary of nucleophiles and electrophiles